MELAS syndrome (Mitochondrial Encephalopathy, Lactic Acidosis and Stroke-like episodes) is one of the most frequent mitochondrial pathologies. Its diagnosis is based on the classic triad of symptoms its acronym stands for and the presence of ragged red fibres. There is currently no curative therapy for MELAS, and treatment focuses on managing complications that affect specific organs and functions. However, some immunonutrients can be used as a therapeutic alternative in patients with MELAS. We present a scientific literature review accompanied by the clinical case of a patient with dementia and seizures admitted to the intensive care unit.
El síndrome de encefalopatía mitocondrial, acidosis láctica y episodios similares a un accidente cerebrovascular (MELAS) es una de las enfermedades mitocondriales más comunes. El diagnóstico se basa en la existencia de las condiciones incluidas en el significado del acrónimo, más la presencia de fibras rojas rasgadas. No existe cura para el síndrome de MELAS, su tratamiento se enfoca en controlar las complicaciones que afectan órganos y funciones específicas. Sin embargo, algunos inmunonutrientes se han utilizado como alternativa terapéutica en pacientes con este síndrome. Presentamos una breve revisión de la literatura en relación con el manejo nutricional del síndrome MELAS en un paciente con demencia y crisis convulsiva, que fue atendido en la Unidad de Cuidados Intensivos.
MELAS syndrome is one of the most frequent mitochondrial pathologies, with an incidence of 10–15 cases per 100,000 people.1,2 It is a maternally inherited neurodegenerative disease, associated with heteroplasmy, and characterised by mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes.3 Despite its acronym, it has a wide spectrum of clinical variability, including seizures, dementia, exercise intolerance, muscle weakness, migraine, sensorineural hearing loss, peripheral neuropathy, recurrent vomiting and short stature.3–5 Although the mitochondrial structure of MELAS syndrome has been known for more than 40 years, the genomic mutation associated with it was not described until 1988.4
MELAS diagnosis is based on the classic triad of symptoms its acronym stands for and the presence of ragged red fibres.4,5 At the molecular level, it is characterised by different mutations in the mitochondrial DNA, the most common of them being the m.3243A>G mutation in the MT-TL1 gene that encodes transfer RNA.3
There is currently no curative therapy for MELAS. Its treatment focuses on managing neurological, cognitive, metabolic, musculoskeletal, gastrointestinal and cardiopulmonary complications in affected organs. Available options mostly involve modulating mitochondrial autophagy, increasing mitochondrial biogenesis, and restoring the nitric oxide (NO) production and nucleotide pools. More recently, potential new treatments have been developed, such as interventions modulating gene expression of the mitochondrial genome, and solid organ and stem cell transplantations.
Nutrient-enriched diets can be used to modulate the immune system, related to inflammation and metabolic pathways. These interventions are known as “immunonutrition”.6 For instance, branched-chain amino acids (BCAAs), glutamine, arginine, nucleotides, increased ω3:ω6 ratio and antioxidants are some of the nutrients modifying such processes. Many of them have been used as a therapeutic alternative in patients with MELAS. We present here the clinical case of a patient with dementia and seizures admitted to the intensive care unit (ICU), and describe the nutritional management of his case, accompanied by a scientific literature review.
CaseWe report the case of a 46-year-old male patient, a carpenter's assistant, with a history of type 2 diabetes mellitus (DM2), diagnosed seven months earlier, under treatment with sulfonylureas and biguanides. Two months before admission to the hospital, he started experiencing tinnitus, bilateral hearing loss and complex visual hallucinations. A month later, he experienced loss of strength and involuntary movement of his left lower leg, accompanied by alteration in speech with incomplete and paraphasic sentences, anorexia and unintentional weight loss of 5kg. On one occasion, he experienced loss of alertness and sphincter relaxation. The clinical picture progressed to an inability to understand and recognise family members, for which he was referred to the hospital.
On physical examination, the patient was alert with normal physiological respiration, but he was not able to understand or repeat what was said to him or to name objects. The examination of the cranial nerves and campimetry by confrontation revealed no alterations, fundus without papilledema, central primary gaze, presence of ductions and versions, isochoric pupils, presence of consensual photomotor reflex and facial symmetry; the remainder was not assessable. Furthermore, the patient showed 3/5 muscle strength in all four extremities, without atrophy or fasciculations, and pectoral, tricipital, patellar and bilateral Achilles tendon stretch reflexes equivalent to +/++++. Other assessments included BP: 125/75mmHg; HR: 76bpm; RR: 18rpm; temp.: 36.2°C; SaO2: 97%; FiO2: 21%: weight: 42kg; height: 155cm; BMI: 17.5kg/m2.
Laboratory tests reported potassium 4.8mEq/l, sodium 132mEq/l, glucose 251mg/dl, leukocytes 14,400mm3, neutrophils 13,210mm3, lymphocytes 880mm3, haemoglobin 14.9g/dl, platelets 327,000mm3. Arterial blood gas: pH 7.42, pCO2 19.5, pO2 86.4, SO2 95.9%, HCO3 12.6, anion gap 18.5, lactate 3.7mmol/l, glucose 255mg/dl. Cerebrospinal fluid (CSF) glucose 90mg/dl, protein 53.9mg/dl, cells 0mm3 and hyperlactatemia.
In the non-contrast head computed tomography (CT), partial calcifications in the subcortical nuclei and areas of bilateral hypodensity that resemble gliosis were observed. Magnetic resonance imaging (MRI) revealed hypointense lesions in T1, hyperintense lesions in T2, FLAIR (fluid-attenuated inversion recovery) at the bilateral temporoparietal level, predominantly on the left, and a hyperintense lesion in the cerebellar lobe. Based on the suspicion of autoimmune encephalitis, treatment started with methylprednisolone boluses, and VGKC antibodies were assessed: anti-LGI1 and anti-NMDA vs. anti-CV2/CRMP5, anti-Hu and anti-Ma2 for detecting encephalitis of paraneoplastic origin. A brain and muscle biopsy for diagnosing MELAS was also performed.
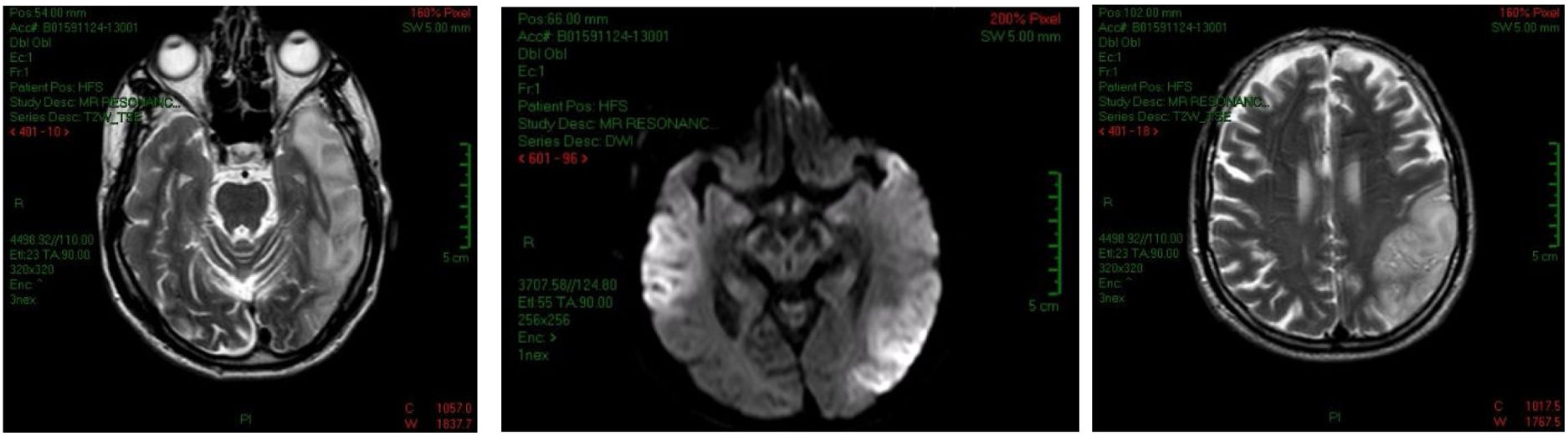
Two days after the examinations, the patient had focal onset seizures that progressed to generalised tonic-clonic seizures, without recovery of alertness, and with significant neurological deterioration, arterial hypotension and asystole. His condition required advanced cardiopulmonary resuscitation and admission to the ICU. In the ICU, he was treated with phenytoin, rapid-acting insulin, and immunonutrition. Ultrasound of the optic nerve sheath showed measures within the expected limits. Echocardiogram provided data supporting heart failure with reduced ejection fraction (30% compensated). Diffusion-weighted MRI control showed stroke-like lesions in the temporal, parieto-occipital regions, predominantly in the left hemisphere, and bilateral symmetric calcifications in the bilateral caudate nuclei (Fig. 1). A left temporal lobe biopsy revealed brain parenchyma with neuronal loss, ischaemic necrosis and spongiosis. Immunohistochemical data showed CD20-negative, CD3-negative and CD68-positive microglia. Genetic testing confirmed m.A3243G mutation in the MTTL1 gene in heteroplasmic state. Immunonutrition was started from the first day: the patient received 19g/day of arginine and 136mg/day of l-carnitine. Once the MELAS diagnosis was confirmed, 1g of IV l-carnitine BID was added. The patient's progress was favourable, and he was extubated, and endoscopic gastrostomy was performed and he was discharged to the ward, where he continued treatment. Upon discharge from the hospital, he was treated with 200mg of carbamazepine TID and 1g of l-carnitine SID.
ReviewThe present case study reports on a critically ill patient with a chronic degenerative disease unknown upon admission. We know that the mutation in MELAS impairs the assembly of protein complexes in the respiratory chain, resulting in impaired mitochondrial energy production, microvascular angiopathy and NO deficiency, which together can impair cerebral vasodilation.3,5 These symptoms can be exacerbated by a compensatory inflammatory response present in critically ill patients.
It is relevant to mention some situations experienced by MELAS patients in contrast to other critically ill patients. Generally, patients in critical condition, depending on the cause, show varying degrees of hypermetabolism and catabolism, while in patients with MELAS in an acute neurocritical phase, as in this case, energy expenditure decreases. In addition, the use of analgesic agents, muscle relaxants, barbiturates and various sedatives further contributes to reducing energy expenditure by 12–32%.7 Some authors have proposed the use of low-carbohydrate or high-fat diets. Unfortunately, the results have shown that these diets cause progressive damage of the muscle fibres.8 Therefore, the use of indirect calorimetry is preferred as a standard for measuring energy expenditure. However, due to the cost and the difficulty in guaranteeing the availability of the equipment, the energy intake is often estimated at 30kcal/kg of ideal body weight or by using various prediction formulas such as the Harris–Benedict equation, suggested by the European Society of Clinical Nutrition and Metabolism, as used in this study.9
Metabolic decompensation, experienced by MELAS patients, can occur for different reasons. It is important to identify the underlying cause in each patient and to monitor the different degrees of catabolism, in order to choose the best treatment and achieve an adequate balance of proteins and nutrients. A safe and effective administration of proteins at 1.0–1.5g/kg of body weight is suggested initially, and should be gradually adjusted depending on the catabolic state.9 Lactate levels may reflect the deterioration of microcirculation and, according to some studies, citrulline (precursor of arginine) can be used as a biomarker of severity in patients with MELAS.10
Immunonutrition plays an important role in critically ill patients due to the beneficial results of its components. BCAAs (leucine, isoleucine and valine) serve as a metabolic source to supplement the needs of skeletal muscle. Glutamine, a conditionally essential amino acid, serves as a metabolic substrate for enterocytes, and reduces bacterial translocation and production of pro-inflammatory cytokines.11 Meanwhile, taurine has the role of maintaining the membrane potential and the intracellular pH, as well as modulating apoptosis (Fig. 2).
Arginine, a conditionally essential amino acid in periods of stress and a precursor of NO, improves the proliferation response of T cells and increases phagocytosis in critically ill patients.12 In MELAS, the energy deficit that stimulates mitochondrial proliferation in the smooth muscle and endothelial cells leads to angiopathy with impaired blood perfusion in the microvasculature of several organs, in addition to a deficiency of NO.3 Oral administration of arginine in patients with MELAS prevents the development of cerebrovascular accidents and reduces the degree of severity of the disease,8,13 while intravenous administration helps to eliminate its main symptoms, such as headache, nausea, vomiting, loss of consciousness and visual impairment.
Some critical patients may have a carnitine deficiency. Carnitine is an amino acid that plays a crucial role in energy production, participates in the long-chain free fatty acid transport into the mitochondria where β-oxidation takes place, and restores intracellular acetyl-CoA groups. Thus, its use in MELAS is well-documented.8,14,15
Nucleotides and ω3 fatty acids are other important components of immunonutrition in critically ill patients.11,12 Nucleotides are essential for the synthesis of RNA, DNA and energy-transporting molecules; their decrease inhibits the function of T cells and macrophages, increasing the risk of sepsis.11 ω3 fatty acids, on the other hand, are a source of energy. They are also components of cell membranes, aid the transport of fat-soluble vitamins, and reduce the synthesis of eicosanoids and inflammatory cytokines, leading to fewer days of mechanical ventilation in critically ill patients, lower prevalence of infections and shorter length of hospital stay.16,17 Based on all of the above, in this case study, immunonutrition was used as a therapeutic strategy from admission to the ICU.
There are other elements of immunonutrition that we have left for the end, because they have been used as specific therapies for MELAS (Table 1). Since in MELAS, energy production is dependent on anaerobic metabolic pathways, there is an elevation of lactate and reactive oxygen species (ROS) levels, causing cell damage and multi-organ dysfunction. Modulation of oxidative stress is thus a therapeutic target.14 Antioxidants, such as ascorbic acid and vitamin E, delay or prevent the oxidation of substrates, limiting stress-induced oxidative degradation and promoting cell survival and resistance.12
Specific treatment for MELAS syndrome.
| Treatment | Recommended doses | Effect |
|---|---|---|
| l-Carnitine | 50–100mg/kg/day | Crucial role in energy production.Improves β-oxidation, restoring the intracellular groups of mitochondrial acetyl-CoA.3,14 |
| l-Arginine | Acute phase: 500mg/kg/day IV or infusion of 10g/m2 in 24h for 3–5 days.Chronic phase: 150–300mg/kg/day.Maximum 500mg/kg/day. | Produces greater availability of NO, improves vasodilation and cerebral blood flow.Reduces the frequency and severity of stroke-like episodes.3,5 |
| Creatine | 0.1–0.15mg/kg/day5g for 14 days, plus 2g of BID for 7 days. | Improves muscle strength and exercise tolerance, reduces free radical production and regenerates ATP in muscles and brain.3,5 |
| Coenzyme Q10Idebenone | 150–300 mg/day or 5–30mg/kg/day divided in 2 doses.Maximum 3000mg | Increases production of mitochondrial ATP, prevents oxidative damage and regenerates other antioxidants, such as vitamin E.Idebenone crosses the blood-brain barrier, having a protective role in stroke-like episodes.5,7,18,19 |
| α-Lipoic acid | Children: 25mg/kgAdults: 50–200mg/dayMaximum 1200mg/day | Antioxidant; increases fat-free mass and total body water, and reduces fat.5,9 |
| Thiamine | 50–100mg/day for 7 daysMaximum 300mg/day | Increases the synthesis of reducing compounds.Reduces lactic acidosis and myopathy.12 |
| Riboflavin | 50–400 mg/day | Precursor of flavoprotein in complex I and II, and cofactor in other enzymatic pathways of β-oxidation and Krebs cycle.5 |
| Ascorbic acid | 1000–2000mg/day | Antioxidant, acceptor of electrons released by the ubiquinone, yielding them to complex IV.5 |
Some cofactors of the respiratory chain, such as succinate, coenzyme Q10, riboflavin, and thiamine – the latter two, components of immunonutrition – have been useful in MELAS treatment.8
Once the diagnosis of MELAS was confirmed, intravenous l-carnitine was added to achieve therapeutic doses. Glycaemic control was achieved with rapid- and intermediate-acting insulin until the patient was discharged, when a DPP-4 inhibitor was prescribed. Drug interactions and their adverse effects are another aspect to consider. Due to a history of DM2, the patient had previously received biguanides. Thus, metformin was contraindicated in this case, as its use, combined with biguanides, can lead to lactic acidosis.
To conclude, there are currently limited interventions to treat MELAS. This is the first case described in the literature in which immunonutrition is used to treat MELAS in an acute phase. Clinical trials on more specific and effective therapies are required, but immunonutrition offers an option both for acute hospital management, as well as a complementary strategy in chronic outpatient management.
Conflict of interestThe authors declare they have no conflict of interest.