Incidence of type 1 diabetes mellitus raises a number of controversies. Our study aim was to contribute to answer the following questions: Is incidence of T1DM increasing? Is age at onset of type 1 diabetes mellitus decreasing? Which are the sex differences? Which are the characteristics in adults?
MethodsA cross-sectional descriptive study using data from a primary source and 3 secondary sources from Navarre collected between 01/01/2009 and 12/31/2016. Annual incidence rates and incidence rate expressed as 100,000 person-years were estimated in the study period by age and sex group. The comparison of the sex and age incidence was made estimating the incidence rate using Poisson's regression methods. The completeness of the register was 96.08%.
ResultsDuring the 8 years analyzed, 428 new cases of type 1 diabetes mellitus were reported (incidence: 8.4/100,000 person-years, 95% CI: 7.6–9.2). Incidence has remained stable and is higher in the group under 15 years old (21.5) than in adults (5.9). Males aged 10–14 years and females aged 5–9 years were the groups with the highest incidence. Incidence then decreased with increasing age. Type 1 diabetes mellitus predominates in males aged 10–45 years, and no changes were seen in age at onset when analyzed by four-year periods.
ConclusionNavarre shows a very high incidence of type 1 diabetes mellitus in childhood and a low incidence in adulthood. Peak incidence is seen earlier in girls, but the disease predominates in males. Neither incidence nor age at onset have changed.
La incidencia de diabetes tipo 1 plantea diversas controversias. Nuestro objetivo consiste en contribuir a responder a las siguientes preguntas: ¿Está aumentando la incidencia? ¿Se adelanta la edad al comienzo? ¿Cuáles son las diferencias entre sexos? y ¿Cuáles son las características en adultos?
MétodosEstudio transversal descriptivo, con los datos obtenidos de una fuente primaria y 3 secundarias, entre el 01/01/2009 y el 31/12/2016, en Navarra. Se estimaron las tasas anuales y la tasa de incidencia, expresada por 100.000 personas-año de riesgo, en el período estudiado, por grupos de edad y sexo. La comparación de la incidencia por sexo y edad se ha realizado estimando la razón de incidencia a partir de métodos de regresión de Poisson. La exhaustividad del Registro fue del 96,08%.
ResultadosDurante estos 8 años, se registraron 428 nuevos casos (incidencia: 8,4/100.000 habitantes-año; IC95%: 7,6-9,2). La incidencia ha permanecido estable y en menores de 15 años (21,5) es mayor que en adultos (5,9). El grupo de edad con mayor incidencia es el de 10 a 14 años en varones y el de 5 a 9 años en mujeres. A partir de aquí, disminuye con la edad. Predomina en hombres entre los 10 y 45 años y, separando por cuatrienios, no hay cambios en la edad al comienzo.
ConclusionesNavarra muestra muy alta incidencia de diabetes tipo 1 en la infancia y baja incidencia en adultos. El pico de incidencia se da antes en las niñas, pero la enfermedad predomina en varones. Ni la incidencia ni la edad al comienzo se han modificado.
Type 1 diabetes mellitus (DM1) is currently a chronic disease with a strong socioeconomic and healthcare impact. It is essential to obtain the most precise information possible about the epidemiology of the disease, in an attempt to identify the underlying etiopathogenesis and adequately channel the available healthcare resources.
Most of the known studies on the epidemiology of DM1 refer to the childhood and adolescent period. Few publications focus on adults, and studies on the incidence of DM1 without age limits are exceptional.1,2 The results in children under 15 years of age reported by the DIAMOND Project Group,3 the EURODIAB group,4 the IDF5 and the different Spanish groups6,7 indicate a more than 350-fold variation in incidence among the world population, a more than 6-fold variation in the European population, and a more than two-fold variation in the Spanish population. In adults, the difficulty in distinguishing DM1 from insulin deprivation type 2 diabetes and latent autoimmune diabetes of adults (LADA), as well as the variable age ranges considered in the different studies, preclude the drawing of firm conclusions.1,2
In any case, the incidence of DM1 is considered to have increased since the late 1980s,3,4,8–10 though not in a linear manner or with equal intensity in different countries,4,11 and appears to have stabilized in the past decade.12,13 Moreover, there are time and geographical variations in the distribution of the disease according to age and gender groups that have given rise to theories about the triggering factors involved in the onset of DM1 (adolescence, body weight, modern lifestyle, environmental factors, etc.).14–16
With regard to age groups, most registries document the highest incidence among individuals between 10 and 14 years of age,17,18 though it is not clear whether the behavior is the same in both genders or whether the patient age at the time of diagnosis is decreasing.14,19,20 In adults, the incidence is lower than in children, and while some authors describe a progressive decrease in incidence with advancing age,2 older studies report different peaks in the course of life.21,22
Unlike what we usually see in autoimmune diseases, diabetes shows a male predominance in adulthood23 and shows a similar incidence in males and females under 14 years of age.16 However, it has been suggested that in high incidence countries, DM1 predominates in boys, while in low incidence countries the disease predominantly affects girls.24
A number of points of controversy therefore persist: Is the incidence of DM1 still increasing or has it stabilized? Has patient age at onset of the disease decreased? What are the gender differences? What characterizes the incidence of DM1 in adults? In order to help answer these questions, the present study offers data on the evolution of the incidence of DM1 in Navarre (Spain) over an 8-year period (2009–2016), with no age limits at the time of diagnosis of the disease. We also describe the characteristics of these patients at baseline, with regard to age and gender.
Material and methodsA descriptive, cross-sectional study was carried out in which information was collected on a prospective basis. All cases diagnosed with DM1 in Navarre between 1 January 2009 and 31 December 2016 were included in the study, excluding those individuals living in the region for less than 6 months. The primary data sources were all the public (n=3) and private (n=2) hospitals in the region of Navarre. The secondary data sources were: (1) primary care centers, through the Clinical Planning and Management Service; (2) the electronic clinical histories shared between primary and specialized care; and (3) the Diabetics Association of Navarre (ANADI). These secondary sources contributed 30 new cases: 10 under 15 years of age (3 aged 0–4 years, 5 aged 5–9 years, and 2 aged 10–14 years); 6 between 15–29 years of age; 7 between 30–44 years of age; and 7 over 45 years of age. The completeness of the registry was assessed based on the capture–recapture method, and was found to be 96.08% (81.37% for the primary data source). At the methodological level, contact with the different sites was made by telephone and e-mail between the study data manager and the study supervisors in each hospital, in primary care and in the ANADI. The reliability of the sources was reinforced by Regional Order 10/2010, of 21 January, creating an electronic file (the Navarre Type 1 Diabetes Registry) including the declaration of all healthcare centers where cases of diabetes are diagnosed or in which information is available on the diagnosis of the disease.
The following diagnostic criteria for DM1 were taken into consideration: (a) positive anti-GAD and/or anti-IA2 antibodies, together with the persistent need for insulin therapy started less than 6 months after diagnosis of the disease; and (b) negative antibodies with specific characteristics referring to the onset of the disease (clinical and laboratory test data: ketosis or ketoacidosis), with the persistent need for insulin therapy started less than 6 months after diagnosis of the disease. Patients with DM1 are monitored in Endocrinology outpatient clinics; accordingly, if insulin treatment can be permanently discontinued over time and the patients are reclassified as having type 2 diabetes, their names are removed from the registry.
Statistical analysisCalculation of the incidence rates was based on the population census data of the region of Navarre (Source: Spanish National Statistics Institute [INE]). The annual rates and incidence rate, expressed per 100,000 person-years of risk, during the study period, were estimated according to age and gender groups. The 95% confidence intervals (95%CI) were estimated assuming an underlying Poisson distribution. The comparison of incidences between the different gender and age groups was made by estimating the incidence rate ratios based on Poisson regression analysis. The study was evaluated and approved by the Research Ethics Committee of Navarre.
ResultsA total of 428 new cases of DM1 were recorded, representing an incidence of 8.4/100,000inhabitants-year. The mean annual population of Navarre in the period 2009–2016 was 640,063 inhabitants.
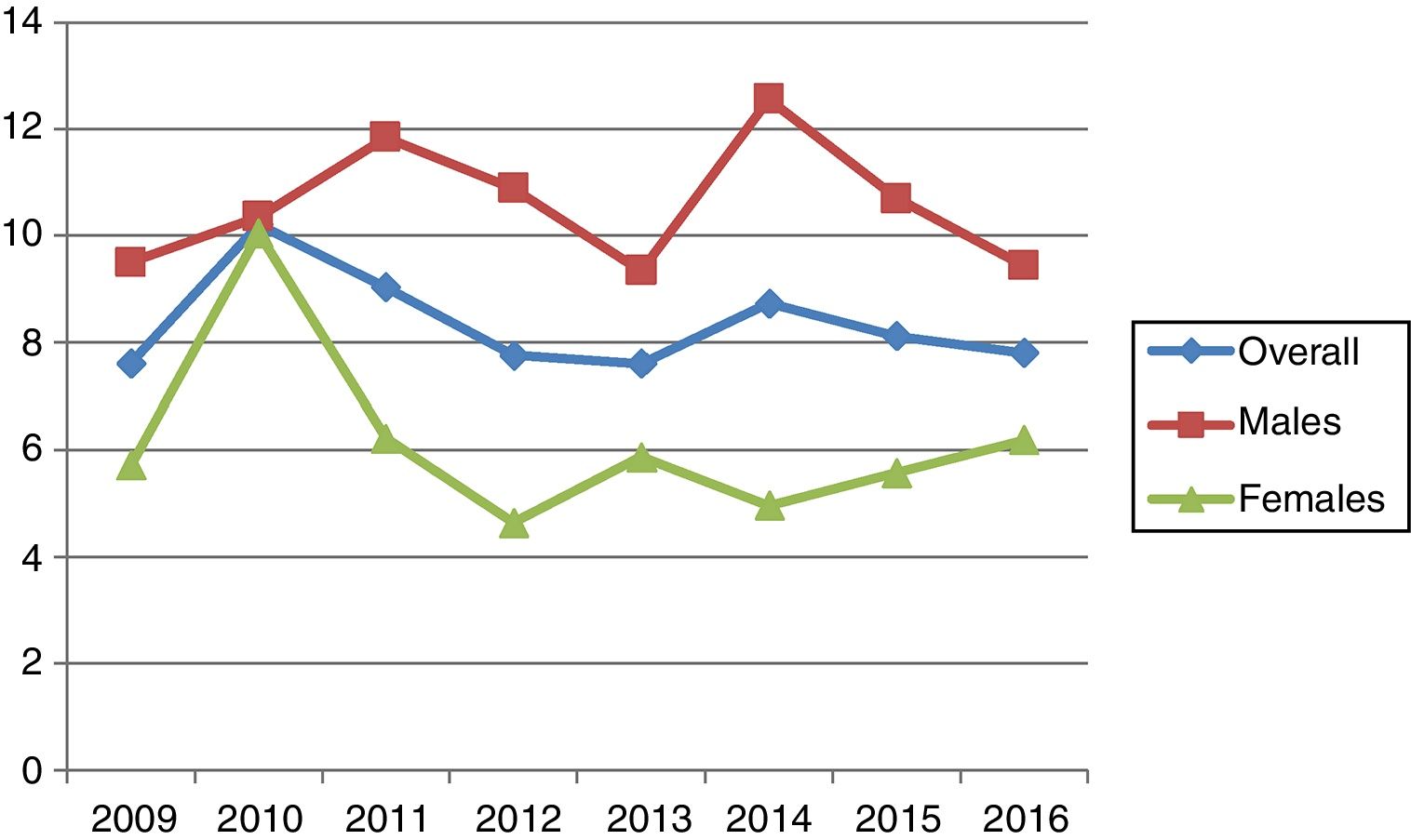
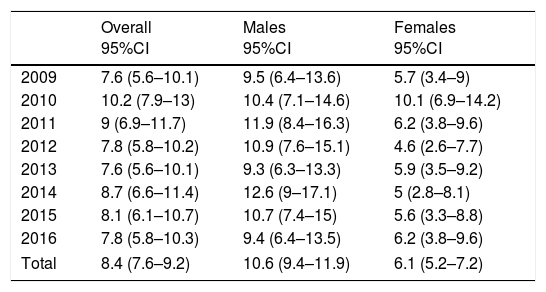
The highest incidence was recorded in 2010 (10.2; 95%CI: 7.9–13) and the lowest in 2009 and 2013 (7.6; 95%CI: 5.6–10.1). During the 8 years of follow-up, the incidence varied but remained stable in both males and females (Table 1 and Fig. 1). The gender distribution was 270 males (63.1%) and 158 females (36.9%). With regard to age distribution, 171 subjects were under 15 years of age (39.9%) while 257 were over 15 years of age (60.1%). The incidence among patients under 15 years of age (incidence rate: 21.5; 95%CI: 18.4–25) was greater than in adults (5.9; 95%CI: 5.2–6.7; p<0.001). However, in absolute terms, the largest number of disease onsets occurred between the ages of 15 and 29 years.
Annual incidence (2009–2016), overall and according to gender.
| Overall 95%CI | Males 95%CI | Females 95%CI | |
|---|---|---|---|
| 2009 | 7.6 (5.6–10.1) | 9.5 (6.4–13.6) | 5.7 (3.4–9) |
| 2010 | 10.2 (7.9–13) | 10.4 (7.1–14.6) | 10.1 (6.9–14.2) |
| 2011 | 9 (6.9–11.7) | 11.9 (8.4–16.3) | 6.2 (3.8–9.6) |
| 2012 | 7.8 (5.8–10.2) | 10.9 (7.6–15.1) | 4.6 (2.6–7.7) |
| 2013 | 7.6 (5.6–10.1) | 9.3 (6.3–13.3) | 5.9 (3.5–9.2) |
| 2014 | 8.7 (6.6–11.4) | 12.6 (9–17.1) | 5 (2.8–8.1) |
| 2015 | 8.1 (6.1–10.7) | 10.7 (7.4–15) | 5.6 (3.3–8.8) |
| 2016 | 7.8 (5.8–10.3) | 9.4 (6.4–13.5) | 6.2 (3.8–9.6) |
| Total | 8.4 (7.6–9.2) | 10.6 (9.4–11.9) | 6.1 (5.2–7.2) |
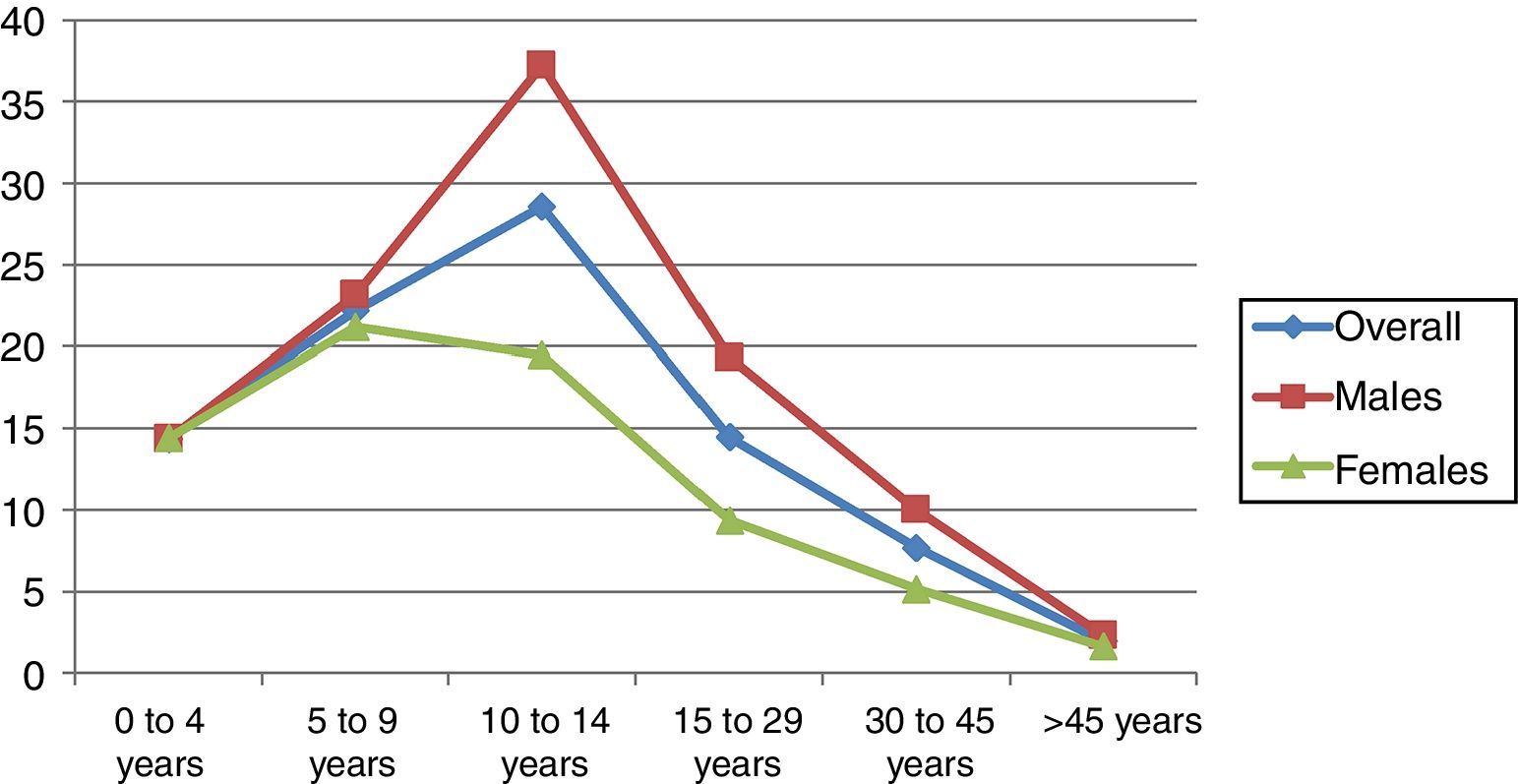
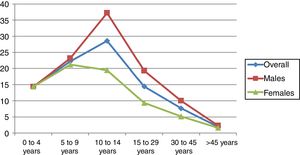
The age group with the highest incidence was 10–14 years in males and 5–9 years in females. The overall incidence in males was higher than in females, at the expense of the between 10 and 45 years age groups (p=0.008–0.001) (Table 2). In adults, the incidence decreased with advancing age in both gender groups (Fig. 2). No decrease in patient age at diagnosis was observed on stratifying the sample into four-year intervals (Table 3).
Incidence according to age and gender (2009–2016).
| Overall 95%CI | Males 95%CI | Females 95%CI | p-Value* | |
|---|---|---|---|---|
| 0–4 years | 14.3 (10.2–19.6) | 14.3 (8.7–22.1) | 14.4 (8.6–22.5) | 0.987 |
| 5–9 years | 22.1 (16.9–28.5) | 23.1 (15.8–32.6) | 21.1 (14–30.6) | 0.737 |
| 10–14 years | 28.5 (22.3–35.9) | 37.2 (27.4–49.3) | 19.4 (12.4–29) | 0.008 |
| 15–29 years | 14.4 (11.9–17.3) | 19.3 (15.3–24) | 9.3 (6.5–12.8) | <0.001 |
| 30–45 years | 7.6 (6.2–9.3) | 10 (7.7–12.7) | 5.1 (3.5–7.3) | 0.002 |
| >45 years | 1.9 (1.4–2.6) | 2.3 (1.5–3.4) | 1.6 (0.9–2.5) | 0.232 |
| Total | 8.4 (7.6–9.2) | 10.6 (9.4–11.9) | 6.1 (5.2–7.2) | <0.001 |
Four-yearly age group comparisons of incidence.
| 2009–2012 95%CI | 2013–2016 95%CI | p-Value | |
|---|---|---|---|
| 0–4 years | 12.3 (7.1–19.7) | 16.4 (10.3–24.9) | 0.372 |
| 5–9 years | 19.7 (12.9–28.9) | 24.4 (16.9–34.1) | 0.415 |
| 10–14 years | 29.5 (20.7–40.9) | 27.6 (19.3–38.2) | 0.769 |
| 15–29 years | 16.3 (12.7–20.6) | 12.4 (9.1–16.4) | 0.145 |
| 30–45 years | 7.9 (5.8–10.4) | 7.4 (5.4–9.9) | 0.747 |
| >45 years | 2 (1.2–3) | 1.8 (1.1–2.8) | 0.830 |
The results of our study confirm a very high incidence of DM1 in children between 5 and 14 years of age in Navarre. The overall incidence, without age limits, has not changed over the last 8 years in either males or females. The peak incidence corresponded to 10–14 years of age in males and 5–9 years in females. From these ages onwards the incidence of the disease was found to decrease. No reduction in patient age at onset of the disease was observed.
Is the incidence of DM1 still increasing or has it stabilized?Over the last four decades the incidence of DM1 among children under 15 years of age in Navarre has clearly increased (4-fold).8 This has caused the region to rise from tenth to fifth position in the Spanish national ranking,6 which is headed by the Canary Islands,25 and to stand above the Spanish average,7 with figures similar to those of the German Registry (22.8).9 Nevertheless, the incidence has remained stable since the year 2000.8 This stability is consistent with the situation found in northern countries12,13 and also in Spain in the case of the province of Biscay,26 and may be due to periodic fluctuations in incidence, though it may also indicate a depletion of genetically susceptible individuals in the areas of increased risk and/or a decrease in environmental determining factors.2 In contrast, in Italy,17 Australia11 and the United States,10 the incidence has continued to increase over the last decade in subjects under 15 years of age. Among individuals between 15 and 29 years of age, the incidence in Navarre (14.46) is the highest in Spain (higher than in Catalonia, the Canary Islands or Badajoz),1 similar to that recorded in West Yorkshire (14.8) and intermediate between the incidences recorded for Sweden (13.8) and Leicestershire (15.3),23 though in this case the data likewise have not changed over the last 8 years, in contrast to the situation found in the United States.10 From 30 years of age onwards, our figures are lower than those recorded in Denmark,21 and here again stabilization has been observed over the last 8 years. In Finland, however, the incidence of the disease in individuals between 15 and 39 years has continued to increase.19 We have found no publications allowing comparisons to be made with the other Spanish regions from 30 years of age onwards.
Has patient age at onset of the disease decreased?In the opening years of this century it was believed especially in Sweden12 and Belgium24 that DM1 was starting to manifest at earlier ages; as a result, there would be no true increase in the incidence of the disease, merely a shift of DM1 toward earlier ages. By contrast, other countries such as Finland, the United Kingdom and Italy defended a true increase in the incidence of the disease.2 In our study, on comparing the incidence of DM1 in the different four-year age groups, no increase in incidence or reduction in age at onset of the disease was observed. The pooled peak incidence in boys and girls continues to correspond to the 10–14 years age group, as in most published studies,5,8,17 though in other settings such as Finland19 and Biscay26 the peak incidence is observed between 5 and 9 years of age. Likewise, in the United States, no earlier age at onset has been recorded,10 and the Swedish registry in turn indicates that the initially observed earlier age at onset has faded.12
What are the gender differences?The most widely held opinion is that the incidence according to gender in children under 15 years of age is similar,16 though after that age and in contrast to what is recorded in most autoimmune disorders, a male predominance is observed.23 It has also been suggested that among individuals under 15 years of age boys predominate in high incidence countries, while girls predominate in low incidence regions.24 In line with this scenario, we observed an increased incidence in males in the 10–14 years of age interval, in concordance with the findings in Hungary18 and Finland.19 The peak incidence in Navarre was seen to occur earlier in girls (5–9 years) than in boys (10–14 years), in concordance with the findings in Sweden, Israel and Australia,14 though the data differ from those recorded in Biscay.26 In line with the data found in the literature,2 we observed a significantly higher incidence in males versus females among those aged 10–45 years.
What characterizes the incidence of DM1 in adults?The incidence of DM1 in adults decreases with age. This decrease is sharper in high incidence areas and more gradual in low incidence areas.2 Our results are consistent with the above, showing a sharp decline in Navarre, a region with a high incidence of DM1 in childhood. No peaks in incidence were seen in our study from 50 years of age onwards, in contrast to the observations of four of the publications on the incidence of DM1 in adults.1,22 To establish the incidence of DM1 in adults with any precision, one has first to overcome a number of obstacles. These include there being few registries advocating the identification of patients of this kind; atypical clinical data in the presentation of DM1; the erroneous classification of DM1 as corresponding to type 2 diabetes; and the scant use of beta cell autoimmune markers for etiological diagnosis in new cases of DM1.2
Our incidence study excluded patients considered to have latent autoimmune diabetes of adults (LADA) according to the three criteria of Fourlanos et al.27 (age at diagnosis>30 years; the presence of anti-islet antibodies; and no need for insulin therapy during at least 6 months after the diagnosis), for although this is a controversial subject, we consider the characteristics of LADA to be different and intend to address such patients in future studies.
As strengths of this study, mention should be made of the fact that this is an incidence registry with no age limit and involving a reliable data collection system, as is shown by the completeness of the registry determined by means of the capture–recapture method.
As limitations of the study, mention should be made of the possibility of our failing to diagnose adults with DM1 because such individuals were regarded as corresponding to type 2 diabetes cases because of their age at onset of the disease; the current follow-up of less than 10 years; and the limited number of cases compared with the national registries of large countries. In Navarre, approximately 440 subjects per 100,000 inhabitants and year develop type 2 diabetes. The existence of the DM1 registry, known to primary care physicians, and the recovery of possible cases of DM1 from the primary care information systems, minimize the first of the aforementioned limitations. With regard to the duration of follow-up, the 8-year period is equal to or longer than the periods contemplated in a number of publications,10,14,17,20,21,24 and although the number of cases of disease onset was not high in absolute terms, the fact that Navarre is a small community makes the figures more precise.
In conclusion, the incidence of DM1 and the patient age at onset of the disease have stabilized in Navarre over the last 8 years. Male predominance between the ages of 10 and 45 years is confirmed. The peak incidence occurs around puberty and then gradually decreases with age, with no younger patient age at disease onset or other posterior incidence peaks being recorded. Large national DM1 registries involving long follow-up periods are needed to better understand the epidemiology of the disorder and the triggering factors, with a view to acting against the appearance of the disease.
Authorship and contributionLuis Forga was involved in the conception and design of the study, interpretation of the data, and drafting of the manuscript.
Ibai Tamayo performed data collection and data analysis and interpretation.
María Chueca contributed to the compilation and interpretation of the data.
Berta Ibáñez contributed to the statistical design and analysis of the data.
Amaya Sainz de los Terreros contributed to the compilation and interpretation of the data.
María José Goñi was involved in the conception and design of the study, interpretation of the data, and drafting of the manuscript.
All authors performed a critical review of the article and approved the final version.
Financial supportThis study was supported by research grants from the Instituto de Salud Carlos III (PI10/02715), the Government of Navarre (53/2008) and the Fundación CAN/La Caixa (Project 28/2014).
Conflicts of interestThe authors state that they have no conflicts of interest.
The authors thank the other members of the Type 1 Diabetes Study Group of Navarre, named below, for their collaboration in this study: Ema Anda, Marta García-Mouriz, Ana Iriarte, Francisco Javier Lafita-Tejedor, María Dolores Ollero, Francisco Javier Pineda, and Rosa Rodríguez-Erdozain (Department of Endocrinology and Nutrition of Complejo Hospitalario de Navarra, Pamplona), Sara Berrade (Section of Pediatric Endocrinology of Complejo Hospitalario de Navarra, Pamplona), Juan Pablo Martínez de Esteban and Marta Toni (Endocrinology, Hospital García Orcoyen, Estella), Francisco Javier Basterra and Patricia Munárriz (Endocrinology, Hospital Reina Sofía, Tudela), and Francisco Javier Escalada (Department of Endocrinology and Nutrition, Clínica Universidad de Navarra).
Please cite this article as: Forga L, Tamayo I, Chueca M, Ibáñez B, Sainz de los Terreros A, Goñi MJ, et al. La incidencia de diabetes tipo 1, en Navarra, se ha estabilizado en los últimos 8 años. Endocrinol Diabetes Nutr. 2018;65:274–279.