Early detection of heterozygous familial hypercholesterolemia (HFH) is needed to prevent premature cardiovascular events. Our aim is to describe the course of an HFH screening detection day in the Northern Cadiz Health Area in Spain and to analyze the data recorded.
Subjects and methodsDescriptive study of an FH cascade screening program. Index cases (ICs) and their 1st and 2nd grade relatives were appointed during a weekend by the FH Foundation. Venous blood samples were taken from the subjects for genetic, blood, and chemistry tests; specialized medical consultation and physical examination were performed.
ResultsThe study sample consisted of 132 subjects: 21 ICs and 111 relatives (16 under 18 years old), with a mean age of 11.4 years (SD 4.57). Mean age of subjects over 18 years was 45.2 years. A gene mutation was found in 90 relatives. Mean age at diagnosis was 25 years (SD 17.7) for relatives and for 36.4 years (SD 17.2; p=.01) for ICs. Smoking rate was higher in relatives than in ICs (26.3% vs 4.8%; p=.02) and corneal arcus was more common in ICs as compared to relatives (47.6% vs 12.6%; p<.001). Prior myocardial infarction was recorded in 14.3% of ICs and 4.2% of relatives respectively (p=.07). Maximum lipid lowering treatment was being administered to 43.1%.
ConclusionsThe screening detection approach identified the estimated 4% population with HFH in the area, and allows for diagnosing HFH 11.4 years earlier.
La detección precoz de la hipercolesterolemia familiar heterocigota (HFH) es necesaria para prevenir eventos cardiovasculares prematuros. Nuestro objetivo es describir el desarrollo de una jornada de detección de HF (JHF) en el Área de Gestión Sanitaria Norte de Cádiz (AGSNC) para su cribado en España, así como analizar los datos obtenidos.
Pacientes y métodosEstudio transversal de una JHF en cascada a la que acudieron los casos índices (CI) diagnosticados genéticamente y sus familiares de primer y segundo grado subsidiarios de presentar HFH. Se analizaron variables clínicas, sociodemográficas y se recogieron muestras biológicas para estudio genético.
ResultadosSe estudiaron 132 sujetos: 21 CI y 111 familiares; 16 eran menores de 18 años, con una edad media de 11,4 años (DE: 4,57). De los mayores de 18 años, el 56% (n=65) fueron mujeres, con una edad media de 45,2 años (DE: 15,9). Noventa familiares eran portadores de una mutación. La edad media de diagnóstico de los familiares fue de 25 años (DE: 17,7), y la de los CI, de 36,4 años (DE: 17,2); p=0,01. El tabaquismo activo fue mayor en los familiares que en los CI (26,3% vs 4,8%; p=0,02) y la presencia de arco corneal en menores de 45 años era más frecuente en los CI (47,6% vs 12,6%; p<0,001). El 14,3% de CI habían presentado un infarto de miocardio vs el 4,2% de los familiares; p=0,07. El 43,1% estaban con máximo tratamiento hipolipemiante oral.
ConclusionesLa estrategia de detección en cribado identificó al 4% de la población estimada con HF del AGSNC. Esta búsqueda activa de HF en los familiares anticipa su diagnóstico en 11,4 años.
Heterozygous familial hypercholesterolemia (HFH) is the most common genetically based cause of early coronary disease, and up to 10% of all subjects with premature coronary events have FH.1,2 In Spain, approximately 15% of the HFH population has suffered an atherosclerotic cardiovascular event.3,4 Heterozygous familial hypercholesterolemia is therefore a public health problem, and its diagnosis and treatment are mandatory. The disease is characterized by an autosomal dominant hereditary pattern and presents in approximately 50% of the offspring of the affected individual.5,6 The prevalence of HFH has been seen to increase with the progressive introduction of detection programs, from 1/500 to 1/200 individuals in the most recent studies.7 By contrast, homozygous familial hypercholesterolemia has a much lower prevalence of approximately 1/450,000 individuals.8 The presence of atherosclerotic cardiovascular disease in HFH can be prevented by the existing lipid-lowering treatment options: statins, ezetimibe9–11 and/or PCSK9 inhibitors that effectively reduce LDL-cholesterol (LDLc) levels, contributing to the achievement of the LDLc objectives9–15 and to the reduction of cardiovascular events in these subjects. A precise tool is also available that quantitatively predicts the risk of a new cardiovascular event in this population,16 though there is still an unmet need to establish an early diagnosis of the disease. Different strategies have been adopted in this regard, such as the approach used in the Netherlands17 or, in the absence of a similar plan in our country, a weekend screening program such as that conducted by the Familial Hypercholesterolemia Foundation (FHF),18,19 which contributes to diagnosis and treatment in order to prevent the development of premature cardiovascular disease (CVD).20 In recent years, economic studies have demonstrated the cost-effectiveness of implementing cascade genetic screening initiatives.21–23 However, family screening is usually performed in families with an established history of CVD and not on a general population basis for familial hypercholesterolemia. A screening program in our health system is therefore needed.
The purpose of this study was to describe the implementation of a familial cascade detection day or session for the population screening of familial hypercholesterolemia (FHS) in a healthcare area in Cádiz (Spain), and to analyze the clinical and laboratory test data for inclusion in the Spanish Familial Hypercholesterolemia Cohort Study (SAFEHEART).
Material and methodsFamilies with HFH from the Área de Gestión Sanitaria Norte de Cádiz (AGSNC), comprising over 450,000 users, were studied. The FHS constitutes a descriptive, cross-sectional study within the previously described SAFEHEART study,19 developed at Hospital de Jerez de la Frontera by the UGC of Internal Medicine and the FHF. Initially, 32 subjects with a score of 6 or more points were identified according to the clinical criteria of the Dutch Lipid Clinic Network (DLCN) as probable index cases (ICs; subjects most likely to have HFH). These individuals subsequently underwent a genetic study, which confirmed the diagnosis of HFH in 22 of them (68.7%).
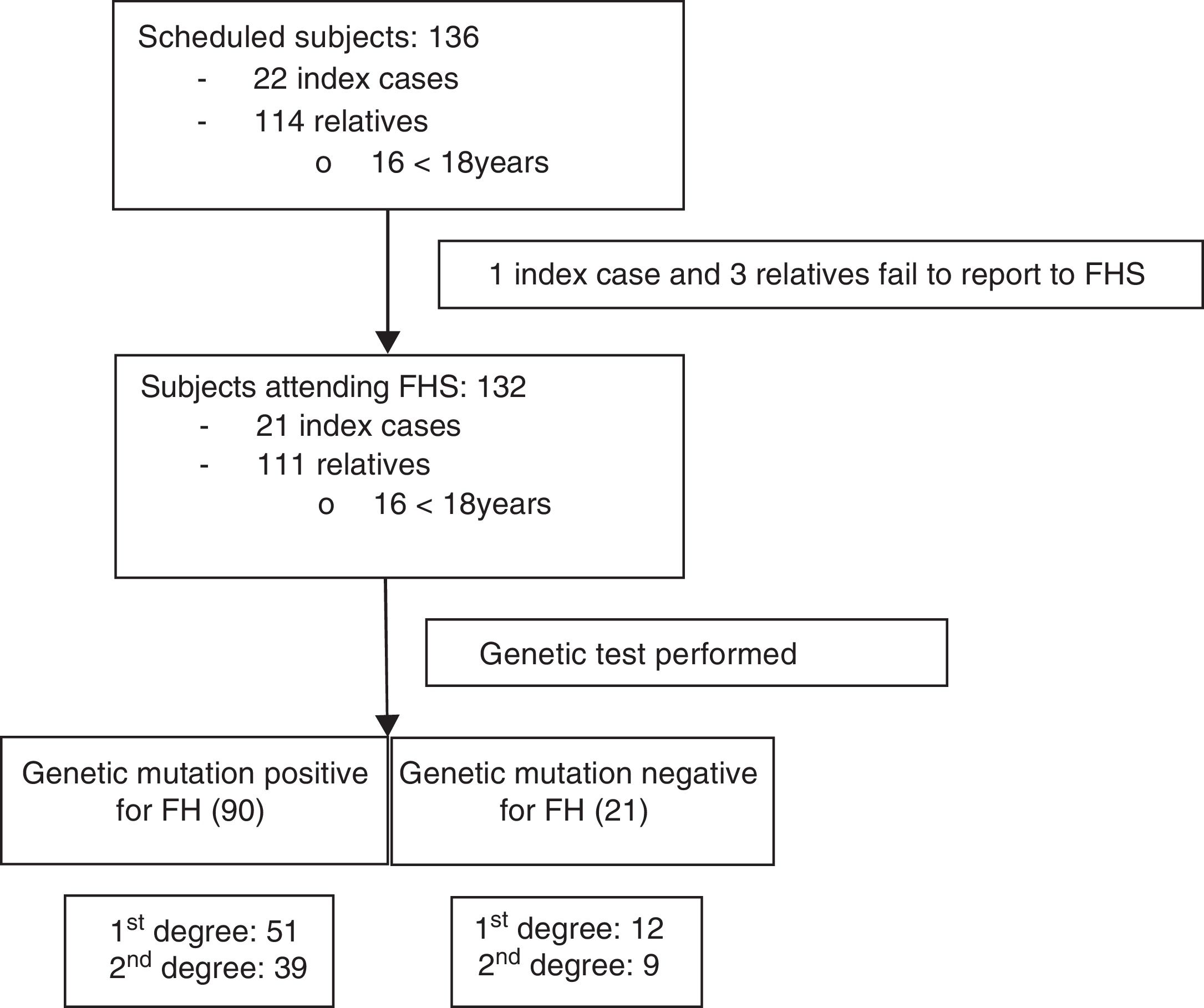
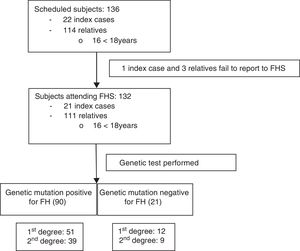
Once informed consent was obtained from the ICs, the FHF made telephone contact and began the information process for both the ICs and their first- and second-degree relatives potentially presenting familial hypercholesterolemia: total cholesterol (TC)>250mg/dl and/or LDLc>190mg/dl in subjects over 18 years of age, or TC>220mg/dl and/or LDLc>150mg/dl in those under 18 years of age (Fig. 1). All of them were scheduled to attend the FHS on 20 and 21 February 2016, with the participation of 6 physicians, two nurses, two laboratory technicians, a biologist, a nutritionist and the cascade screening coordinator. All subjects attending the FHS were included in the study.
The FHS was conducted in four phases: patient receipt, blood sampling, medical consultation, and dietary survey. During reception, information was provided about HFH, as well as healthy lifestyle habits. Venous blood sampling was performed after 12h of fasting for all subjects over 11 years of age, with sample collection in two 9-ml EDTA tubes, a 9-ml GelBond® serum tube and two 4-ml citrate tubes. The serum, plasma and DNA samples were aliquoted and stored at −80°C in a biobank at the core laboratory of the Cardiovascular Research Institute Hospital Sant Pau (Barcelona, Spain).15 The subjects under 11 years of age underwent genetic testing in saliva, and the biochemical data were collected from previous test reports provided by the parents. The methodology used for molecular detection has been previously described,24 and the APOB, PCSK9 and LDL receptor genes were analyzed.
During the medical visit, the case report form was completed for each subject, and a physical examination was made, in which the following were measured: weight, height, waist circumference, the presence of corneal arcus and tendon xanthomas, as well as blood pressure on two occasions with an Omron® M3 apparatus. Lastly, the patients completed several questionnaires; physical activity was measured using the short version of the International Physical Activity Questionnaire (IPAQ)25; quality of life was assessed using the EuroQoL Group EQ-5D (1990)26; health status was evaluated; and the London School of Hygiene and Tropical Medicine survey was administered.
The present study was approved by the Clinical Research Ethics Committee-FJD (Fundación Jiménez Díaz), and all participants gave written informed consent to both the genetic study and inclusion in the SAFEHEART study. In the case of minors, the legal representative gave the required consent. The FHS is part of the campaign for identifying new HFH cases promoted by the FHF, and has been carried out in Spain since the year 2004.19
Treatment intensityThe intensity of therapy was defined by groups based on its LDLc lipid-lowering potency. Accordingly, low potency was defined as treatment affording reductions of <30%; moderate potency as reductions of 30–50%; and high potency, between 50 and 60%, as reductions of >60%27 (Table 1).
Classification of lipid-lowering treatment according to the intensity of LDLc reduction.
| Low intensity: LDLc reduction <30% |
| Simvastatin 10mg |
| Pravastatin 10–20mg |
| Lovastatin 10–20mg |
| Fluvastatin 40mg |
| Pitavastatin 1mg |
| Ezetimibe 10mg |
| Moderate intensity: LDLc reduction 30–50% |
| Atorvastatin 10–20mg |
| Rosuvastatin 5–10mg |
| Simvastatin 20–40mg |
| Pravastatin 40mg |
| Lovastatin 40mg |
| Fluvastatin 80mg |
| Pitavastatin 2–4mg |
| Simvastatin 10mg+Ezetimibe 10mg |
| Pravastatin 20mg+Ezetimibe 10mg |
| Lovastatin 20mg+Ezetimibe 10mg |
| Fluvastatin 40mg+Ezetimibe 10mg |
| Pitavastatin 1mg+Ezetimibe 10mg |
| High intensity: LDLc reduction 50–60% |
| Atorvastatin 40–80mg |
| Rosuvastatin 20–40mg |
| Simvastatin 20–40mg+Ezetimibe 10mg |
| Pravastatin 40mg+Ezetimibe 10mg |
| Lovastatin 40mg+Ezetimibe 10mg |
| Fluvastatin 80mg+Ezetimibe 10mg |
| Pitavastatin 2–4mg+Ezetimibe 10mg |
| Atorvastatin 20–40mg+Ezetimibe 10mg |
| Rosuvastatin 5–10mg+Ezetimibe 10mg |
| Very high intensity: LDLc reduction >60% |
| Atorvastatin 40–80mg+Ezetimibe 10mg |
| Rosuvastatin 10–20mg+Ezetimibe 10mg |
Data analysis was performed using the SPSS version 23 statistical package (Chicago, IL, USA). The descriptive study of qualitative variables was based on the number of cases and percentages, while quantitative variables with a normal distribution were reported as the mean and standard deviation, and those with a non-normal distribution were reported as the median and interquartile range (IQR). The chi-squared test was used to compare general qualitative variables, while the Student t-test was applied for quantitative variables. The magnitude of association was estimated based on the odds ratio (OR), with a 95% confidence interval (CI). A multivariate analysis was likewise performed. Statistical significance was considered for p<0.05.
ResultsA total of 136 subjects from the Área de Gestión Sanitaria Norte de Cádiz (AGSNC) were enrolled in the study. Twenty-two were ICs and 114 were relatives, with an average of about five relatives per IC. Four cited people did not attend the FHS and were excluded (one IC and three relatives) (Fig. 1). Of the 132 subjects who attended the FHS, 16 were under 18 years of age, with a mean age of 11.4 years (SD: 4.57) (12 males; 75%). Of the subjects over 18 years of age, 56% (n=65) were women, with a mean age of 45.2 years (SD: 15.9).
The mean age at diagnosis for the relatives was 25 years (SD: 17.7), versus 36.4 years (SD: 17.2) in the case of the ICs (p=0.01). In 41.4% of the participants over 18 years of age, the clinical diagnosis of HFH was made by the specialist (DLCN score ≥6); however, subsequent control was performed by the primary care physician in 53.4% of the cases. In turn, 44.7% (n=59) had a family history of early CVD (before 55 years of age in men and 60 years in women); 71.2% (n=42) corresponded to coronary events; 45.8% (n=27) to cerebrovascular events (10 of them also with coronary involvement); and 1.7% (n=1) to peripheral vascular disease.
In the FHS, 90 relatives (81%) were found to have a positive HFH mutation; of these, 56.7% were first-degree relatives (n=51). No mutations were detected in 21 relatives. Of the 111 positive cases (84%), a mutation for RLDL was detected in 83.8% (n=93); 63% corresponded to defective allele mutations and 32% to null allele. A new gene mutation (c.899G>C) of undetermined significance was described in two subjects. In turn, 16.2% of the subjects had a mutation of the APOB gene (n=18), with 100% being defective (7 of them moreover simultaneously presented another mutation of the RLDL gene).
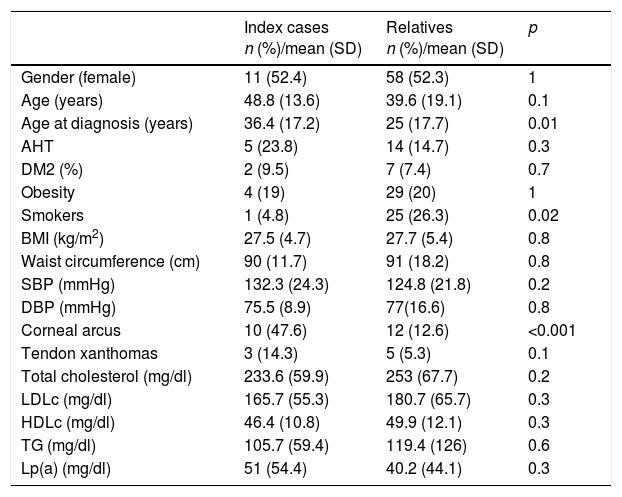
In the case of those over 18 years of age, 19% presented corneal arcus before 45 years of age (n=22), 6.9% presented tendon xanthomas (n=8), 16.4% had arterial hypertension (n=19), and 7.8% were diabetic (n=9). The general characteristics of the ICs and relatives are shown in Tables 2 and 3 (under 18 years of age). Table 4 shows the personal history of CVD and age at the occurrence of events.
General characteristics and lipid profile of the patients included in the study.
| Index cases n (%)/mean (SD) | Relatives n (%)/mean (SD) | p | |
|---|---|---|---|
| Gender (female) | 11 (52.4) | 58 (52.3) | 1 |
| Age (years) | 48.8 (13.6) | 39.6 (19.1) | 0.1 |
| Age at diagnosis (years) | 36.4 (17.2) | 25 (17.7) | 0.01 |
| AHT | 5 (23.8) | 14 (14.7) | 0.3 |
| DM2 (%) | 2 (9.5) | 7 (7.4) | 0.7 |
| Obesity | 4 (19) | 29 (20) | 1 |
| Smokers | 1 (4.8) | 25 (26.3) | 0.02 |
| BMI (kg/m2) | 27.5 (4.7) | 27.7 (5.4) | 0.8 |
| Waist circumference (cm) | 90 (11.7) | 91 (18.2) | 0.8 |
| SBP (mmHg) | 132.3 (24.3) | 124.8 (21.8) | 0.2 |
| DBP (mmHg) | 75.5 (8.9) | 77(16.6) | 0.8 |
| Corneal arcus | 10 (47.6) | 12 (12.6) | <0.001 |
| Tendon xanthomas | 3 (14.3) | 5 (5.3) | 0.1 |
| Total cholesterol (mg/dl) | 233.6 (59.9) | 253 (67.7) | 0.2 |
| LDLc (mg/dl) | 165.7 (55.3) | 180.7 (65.7) | 0.3 |
| HDLc (mg/dl) | 46.4 (10.8) | 49.9 (12.1) | 0.3 |
| TG (mg/dl) | 105.7 (59.4) | 119.4 (126) | 0.6 |
| Lp(a) (mg/dl) | 51 (54.4) | 40.2 (44.1) | 0.3 |
HDLc: high-density lipoprotein cholesterol; LDLc: low-density lipoprotein cholesterol; DM: diabetes mellitus; AHT: arterial hypertension; BMI: body mass index; Lp(a): lipoprotein (a); DBP: diastolic blood pressure; SBP: systolic blood pressure; TG: triglycerides.
Age and gender include subjects over and under 18 years of age.
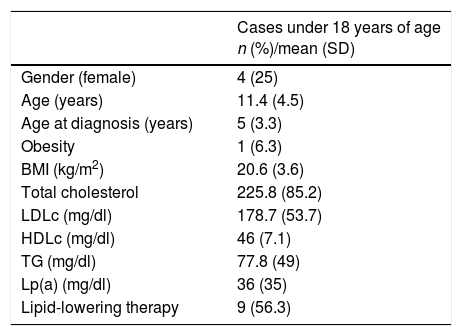
General characteristics and lipid profile of patients under 18 years of age.
| Cases under 18 years of age n (%)/mean (SD) | |
|---|---|
| Gender (female) | 4 (25) |
| Age (years) | 11.4 (4.5) |
| Age at diagnosis (years) | 5 (3.3) |
| Obesity | 1 (6.3) |
| BMI (kg/m2) | 20.6 (3.6) |
| Total cholesterol | 225.8 (85.2) |
| LDLc (mg/dl) | 178.7 (53.7) |
| HDLc (mg/dl) | 46 (7.1) |
| TG (mg/dl) | 77.8 (49) |
| Lp(a) (mg/dl) | 36 (35) |
| Lipid-lowering therapy | 9 (56.3) |
LDLc: low-density lipoprotein cholesterol; HDLc: high-density lipoprotein cholesterol; BMI: body mass index; Lp(a): lipoprotein (a); TG: triglycerides.
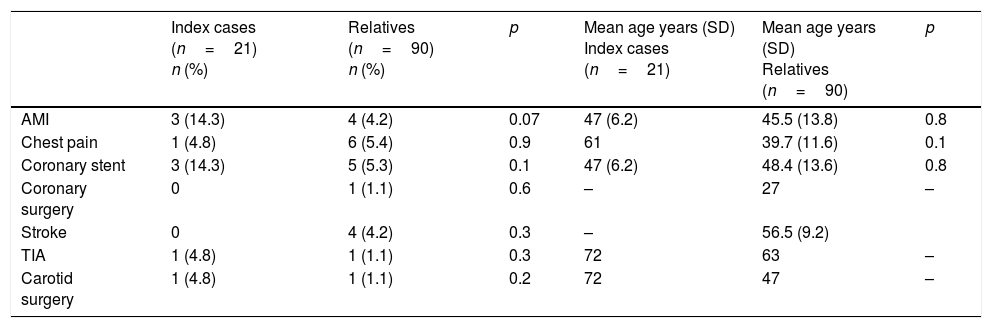
Personal history of cardiovascular disease in patients with heterozygous familial hypercholesterolemia.
| Index cases (n=21) n (%) | Relatives (n=90) n (%) | p | Mean age years (SD) Index cases (n=21) | Mean age years (SD) Relatives (n=90) | p | |
|---|---|---|---|---|---|---|
| AMI | 3 (14.3) | 4 (4.2) | 0.07 | 47 (6.2) | 45.5 (13.8) | 0.8 |
| Chest pain | 1 (4.8) | 6 (5.4) | 0.9 | 61 | 39.7 (11.6) | 0.1 |
| Coronary stent | 3 (14.3) | 5 (5.3) | 0.1 | 47 (6.2) | 48.4 (13.6) | 0.8 |
| Coronary surgery | 0 | 1 (1.1) | 0.6 | – | 27 | – |
| Stroke | 0 | 4 (4.2) | 0.3 | – | 56.5 (9.2) | |
| TIA | 1 (4.8) | 1 (1.1) | 0.3 | 72 | 63 | – |
| Carotid surgery | 1 (4.8) | 1 (1.1) | 0.2 | 72 | 47 | – |
TIA: transient ischemic attack; AMI: acute myocardial infarction; Stroke: cerebrovascular accident.
A total of 88.8% of the patients over 18 years of age (n=103) had received hygiene-dietary recommendations, though only 31% (n=36) claimed to always adhere to these recommendations, while 50% (n=58) did so sometimes. In turn, 81% (n=94) received drug therapy with statins to lower their cholesterol levels. A total of 27.6% were also treated with ezetimibe 10mg (n=32), with a significant difference in ezetimibe use between ICs and relatives (OR: 4.9, 95%CI: 1.8–13.3; p=0.001). A multivariate analysis comparing the lipid profile of positive and negative relatives according to treatment intensity detected no significant differences. A total of 43.1% were receiving the maximum lipid-lowering therapy available for reducing LDLc>50% at the time of recruitment; 16 were ICs with an OR of 5.7 (95%CI: 1.9–17; p<0.001) versus the relatives. Table 5 shows the lipid-lowering potency of the treatment received by the cases over 18 years of age in the study. With regard to lipid-lowering therapy in the patients under 18 years of age, 6 were being treated (37.5%): 5 with statins and one with resins.
Lipid-lowering treatment in index cases and relatives according to lipid-lowering potency (see Table 1).
| Lipid-lowering treatment potency | Index cases (n=21) n (%) | Relatives (n=94) n (%) | p |
|---|---|---|---|
| No treatment | 3 (14.3) | 34 (36.2) | 0.05 |
| Moderate | 2 (9.5) | 26 (27.7) | 0.08 |
| High | 10 (47.6) | 21 (22.3) | 0.01 |
| Very high | 6 (28.6) | 13 (13.8) | 0.1 |
Only two patients without CVD reached the LDLc objective of <100mg/dl as indicated by the guidelines, and none of the patients with a history of CVD reached LDLc <70mg/dl.
DiscussionHeterozygous familial hypercholesterolemia is underdiagnosed in Spain. Accordingly, in the absence of a national screening program, the described genetic cascade screening strategy has been shown to be an effective way of increasing the detection of patients with HFH. During the detection session, 90 new cases of HFH were genetically diagnosed, 16 of which corresponded to individuals under 18 years of age. Bearing in mind that the AGSNC recruitment population consists of approximately 450,000 inhabitants and that the prevalence of the disease is approximately one case per 200 inhabitants, 4% of the estimated subjects with HFH in this area were diagnosed in the course of a weekend.
Therefore, the conduction of such a screening day or session represents an advance in the rapid detection and early diagnosis of these subjects, with genetic cascade screening having been shown to be cost-effective in a recent study.21 Despite all the work involved in subject-by-subject contact through the families, the time required for family screening and the full study of 132 patients is much shorter than if the diagnosis were established incidentally in clinical consultation.
In coincidence with the general population with HFH, the age at diagnosis is late considering that the indication to start lipid-lowering treatment in these patients may be established from 8 to 10 years of age according to the LDLc levels and other cardiovascular risk factors.7 Such active screening for HFH has lowered the age at diagnosis by more than 10 years as compared to previous series,10,11 and the age at diagnosis among relatives was 11.4 years younger than in the case of the ICs (the difference being statistically significant).
On the other hand, CVD is more common in ICs than in the relatives, particularly with regard to myocardial infarction (with significant differences). This may be explained by the incidental finding of HFH in these subjects once they have already suffered an early cardiovascular event, or because premature CVD is one of the criteria used for establishing the diagnosis. On comparing the lipid profile of the ICs versus the relatives, lower TC, triglyceride, HDLc and LDLc levels were found (though without reaching statistical significance), possibly because the ICs were receiving a more intense lipid-lowering treatment than the undiagnosed subjects.
Another relevant finding is that there were significantly fewer smokers among the ICs than in the relatives diagnosed with HFH, presumably due to the increased presence of CVD in the former, with a correspondingly greater awareness of the adverse effects of smoking. A larger number of ICs with FHF had corneal arcus before 45 years of age as compared to the relatives, possibly due to older age among the former.
Among the limitations of the study, it should be noted that the screening of relatives was made among those with LDLc>190mg/dl in adults and >150mg/dl in individuals under 18 years of age. This cut-off point in LDLc concentration was used to make optimum use of the cost of the cascade screening diagnosis; as a result, some relatives with lower LDLc levels might not have been diagnosed at that time.
ConclusionEarly detection and genetic cascade screening are necessary for the prevention of premature CVD in these patients. This strategy diagnosed approximately 4% of the familial hypercholesterolemia population of the AGSNC, and the active search for cases in relatives anticipated diagnosis by 11.4 years, thus contributing to treatment being started earlier.
Financial supportThe present study was funded by the Familial Hypercholesterolemia Foundation (FHF); the Instituto de Salud Carlos III (grant G03/181 and FIS PI12/01289); and the National Cardiovascular Research Center (grant 08-2008).
AuthorshipP. Rubio-Marín: Conception and design of the study, data collection, analysis and interpretation of data, and drafting, review and approval of the submitted manuscript.
A. Michán-Doña: Conception and design of the study, data collection and drafting, review and approval of the submitted manuscript.
J. Maraver-Delgado: Data collection.
R. Arroyo-Olivares: Data collection, analysis, and interpretation.
R. Barrado-Varea: Data collection.
L. Pérez de Isla: Data collection.
P. Mata: Conception and design of the study, data collection and drafting, review and approval of the submitted manuscript
Conflicts of interestThe authors state that they have no conflicts of interest.
Thanks are due to Teresa Pariente for her important work in the FHF–without which the FHS could not be carried out–as well as to Ángela Cabrera de la Calle and María Ángeles Muñoz-Arenillas Calbo, nurses from the UGC of Internal Medicine (Hospital de Jerez de la Frontera), for their active collaboration in the FHS, and to the families with familial hypercholesterolemia for their contribution.
Please cite this article as: Rubio-Marín P, Michán-Doña A, Maraver-Delgado J, Arroyo-Olivares R, Barrado Varea R, Pérez de Isla L, et al. Programa de cribado en cascada para la detección de la hipercolesterolemia familiar. Endocrinol Diabetes Nutr. 2018;65:280–286.