The aim of this study was to determine whether there are differences in salivary oxidative stress between patients with diabetes mellitus type 2 (DM2) and healthy non-diabetic patients, and whether this oxidative stress is associated with the presence of periodontal disease in diabetic patients.
Material and methodsThis observational study included 70 patients divided into three groups according to metabolic control levels: 19 non-diabetic patients (control group); 24 patients with good metabolic control (HbA1c<7%), and 27 patients DM2 with poor metabolic control (HbA1c>7%). The following oxidative stress parameters were measured in all subjects: glutathione peroxidase (GPx), glutathione reductase (GRd), reduced glutathione (GSH) and oxidized glutathione (GSSG). Periodontal health was determined by means of the community periodontal index (CPI) recommended by the WHO.
ResultsThe diabetic group with good metabolic control showed a significant increase in GPx and GRd activity in comparison with the control group (p<0.001). The activity of the enzymes measured was significantly less in patients with poor metabolic control in comparison with the control group and well-controlled diabetic groups (p<0.001). Both diabetic groups showed higher GSSG/GSH quotients and CPI in comparison with the control group, and both parameters were significantly higher in diabetic patients with poor metabolic control in comparison with well-controlled diabetic patients.
ConclusionsPoor metabolic control in DM2 patients is associated with higher levels of salivary oxidative stress and worse periodontal health.
Nuestro objetivo fue analizar si existen diferencias en los niveles de estrés oxidativo salival de pacientes con DM2 en comparación con sujetos sanos no diabéticos, y si dicho estrés oxidativo se puede asociar a la presencia de enfermedad periodontal en pacientes con diabetes.
Material y métodosSe realizó un estudio observacional que incluyó 70 pacientes, estableciéndose 3 grupos de estudio en función del control metabólico: 19 pacientes sin diabetes (grupo control); 24 pacientes DM2 con buen control metabólico (HbA1c<7%), y 27 pacientes DM2 con mal control metabólico (HbA1c>7%). En todos ellos se midieron los siguientes parámetros de estrés oxidativo salival: glutatión peroxidasa (GPx), glutatión reductasa (GRd), glutatión reducido (GSH) y glutatión oxidado (GSSG). El estado de salud periodontal se determinó mediante el índice periodontal comunitario (CPI), recomendado por la OMS.
ResultadosEl grupo de diabetes con buen control metabólico mostró un incremento significativo en la actividad de GPx y GRd con respecto al grupo control (p<0,001). La actividad de dichas enzimas fue significativamente menor en los pacientes con diabetes con mal control metabólico en comparación con el grupo control y de diabéticos bien controlados (p<0,001). Los 2 grupos de pacientes con diabetes mostraron mayor cociente GSSG/GSH e índice CPI con respecto al grupo control, resultando también ambos parámetros significativamente aumentados en el grupo de diabetes con mal control metabólico respecto a los bien controlados.
ConclusionesUn peor control metabólico se asocia a mayores niveles de estrés oxidativo en saliva de pacientes con DM2, así como a un peor estado de salud periodontal.
Periodontal diseases are a group of chronic inflammatory conditions caused by bacteria present in the subgingival biofilm, which affect the tooth-supporting tissues and induce tissue destruction with the formation of periodontal pockets and bone resorption. The ultimate consequence is clinical attachment loss and the loss of teeth.1 Periodontal diseases are currently regarded as chronic infections of the oral cavity that can activate the host immune and inflammatory responses at both the local and the systemic level.2 It is known that periodontal diseases exert an important influence upon the pathogenesis of many systemic diseases, including diabetes mellitus.3 The scientific basis for the association between diabetes mellitus and periodontitis was established in the mid-1990s, and since then periodontitis has been referred to as the «sixth complication of diabetes».4 The current scientific evidence points to the existence of a bidirectional relationship between diabetes mellitus and periodontal diseases. In this sense, diabetes mellitus is associated with an increase in the incidence and progression of periodontitis, while periodontal infection in turn is associated with poorer blood glucose control in diabetic individuals.5
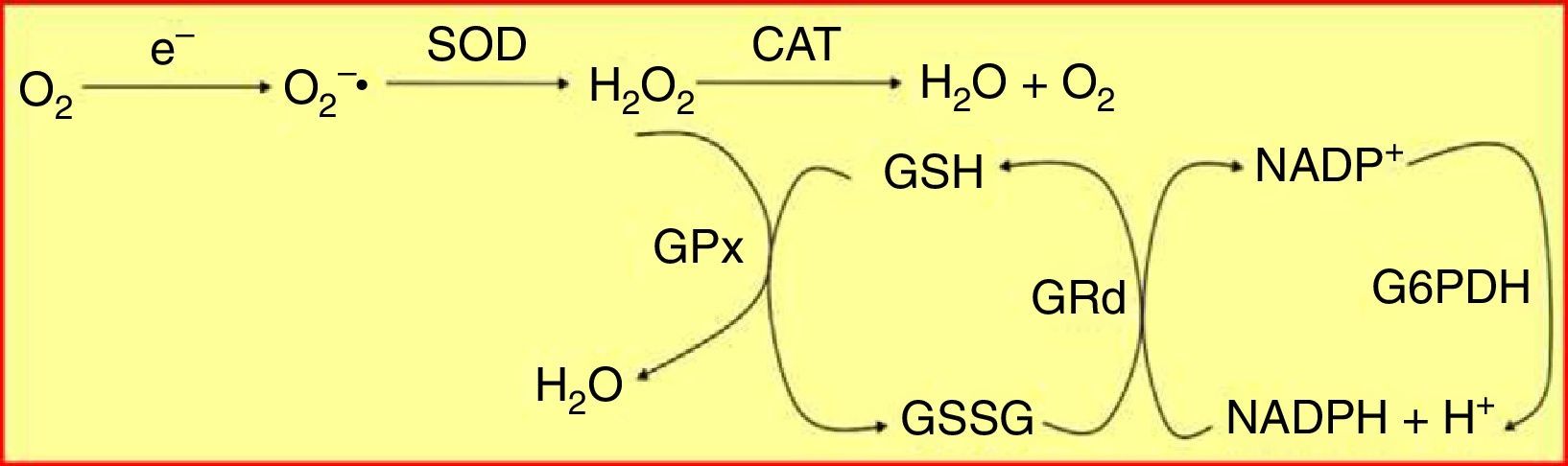
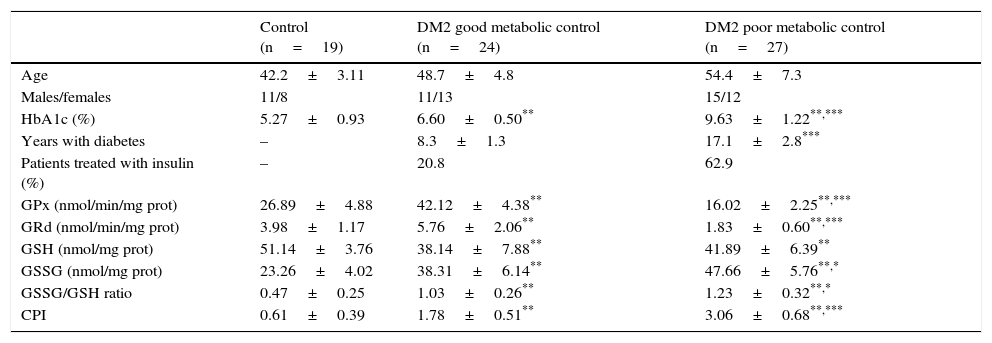
Oxidative stress plays a key role in the physiopathology of the chronic inflammatory process that characterizes both periodontal diseases and diabetes mellitus.5,6 Different antioxidant defence systems contribute to protect the cells from damage caused by free radicals. The glutathione system, which quantitatively constitutes the main antioxidant defence system of the cell, comprises a specific and varied pool of enzymes that contributes to the maintenance of cellular homeostasis against oxidative attack. These enzymes include glutathione peroxidase (GPx) and glutathione reductase (GRd),7 which act and interrelate as is shown in Fig. 1.
The glutathione peroxidase/glutathione reductase (GPx/GRd) enzyme system. Glutathione is mainly found in its reduced state (GSH) and to a much lesser extent in its oxidized state (GSSG). GRd forms together with GPx the glutathione-dependent antioxidant machinery of the body. This system operates cyclically, i.e. the GSH that oxidizes GPx to neutralize H2O2 is in turn reduced by GRd, using NADPH as a cofactor.
CAT: catalase; G6PDH: glucose-6-phosphate dehydrogenase; H2O2: hydrogen peroxide; NADPH: reduced nicotinamide adenine dinucleotide phosphate; O2−: superoxide anion radical; SOD: superoxide dismutase.
From the clinical perspective, the increased incidence of periodontal diseases in diabetic patients has been linked to increased levels of serum oxidative stress in these individuals, secondary to an excessive production of reactive oxygen species (ROS) and a decrease in the host antioxidant defence mechanisms.8 Few studies have addressed the oxidative stress levels and the antioxidant capacity of the saliva of diabetic patients, and those available have used very different methods and reported a great variability in the parameters measured.
The objectives of this study were: (i) to analyze the potential differences in salivary oxidative stress levels between patients with type 2 diabetes mellitus (T2DM) and healthy subjects without diabetes, and to analyze whether such oxidative stress correlates to metabolic control of the disease; and (ii) to assess whether the presence of periodontal disease in patients with T2DM is associated with increased salivary oxidative stress.
Patients and methodsThe study was conducted at the dental school of the University of Granada (Granada, Spain) in collaboration with the department of endocrinology and nutrition of Virgen Macarena University Hospital, and with the participation of the diabetes unit. The project was approved on 7 October 2015 by the ethics committee of the University of Granada.
ParticipantsSubjects diagnosed with T2DM and healthy controls aged 18–65 years who attended the dental clinic for special patients of the dental school of the University of Granada were enrolled into the study. All the subjects were outpatients and gave their prior written informed consent to participate in the study. Patients with type 1 diabetes mellitus (T1DM) or other less common forms of diabetes, pregnant women, active smokers, patients with oral mucosal lesions, and those given antibiotic treatment in the 30 days prior to oral examination and salivary sample collection were excluded from the study.
The total sample of 70 patients was divided into three groups based on the degree of metabolic control, as evidenced by glycosylated hemoglobin (HbA1c), measured on the day of the first visit, irrespective of periodontal status:
- •
Control group: 19 patients without diabetes or prediabetes (HbA1c<5.7%) and randomized to the study from their first visit to the Dental School of the University of Granada. This group comprised patients both with and without periodontal disease who were seen for any other type of dental treatment (tartrectomy, fillings, prosthetic restorations).
- •
Group of diabetics with good metabolic control: 24 patients with DM2 and HbA1c<7%.
- •
Group of diabetics with poor metabolic control: 27 patients with DM2 and HbA1c>7%.
A saliva sample was taken from all patients to measure the following oxidative stress parameters: GPx, GRd, reduced glutathione (GSH), and oxidized glutathione (GSSG). A thorough oral and dental examination was made in all three groups, including periodontal examination. The processing of saliva samples for the quantification of oxidative stress was carried out at the Scientific Instrument Center of the University of Granada.
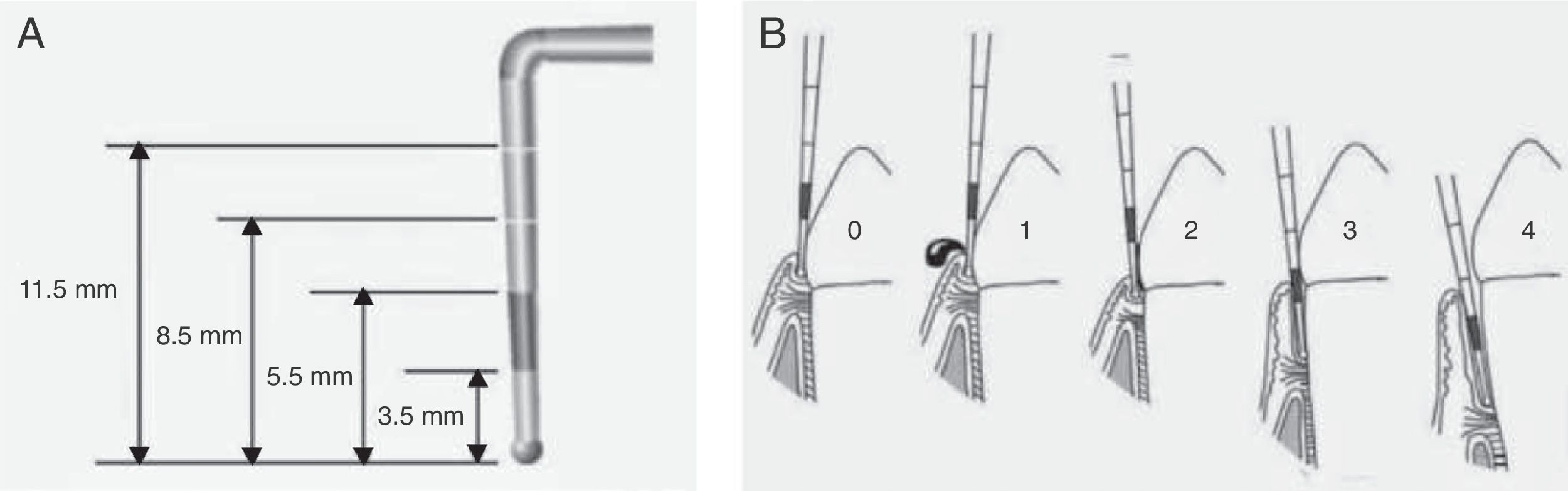
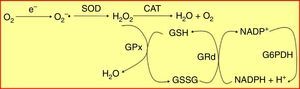
Evaluation of periodontal statusThe evaluation of periodontal health was based on the Community Periodontal Index (CPI), validated by the World Health Organization (WHO). This measures the presence of gingival bleeding, calculus or periodontal pockets, as well as attachment loss and dental loss.9 A specifically designed probe was used for the evaluation – the WHO periodontal probe with a ball tip measuring 0.5mm in diameter – with black marking of the zone between 3.5 and 5.5mm, and two additional marks at 8.5 and 11.5mm (Fig. 2A). To measure periodontal pocket depth and calculate the CPI, the mouth was divided into six segments representing the basic examination units. Dental sextant recording was made after an examination of the first and second molars in the posterior sector and a central tooth in the anterior sector (10 index teeth). The codes for recording the periodontal condition based on this index are shown in Fig. 2B.
Determination of the Community Periodontal Index (CPI). The WHO periodontal probe, which has a ball tip and black marking of the zone between 3.5 and 5.5mm, and two additional marks at 8.5 and 11.5mm, should be used (A). Each sextant is evaluated based on the following codes: 0: healthy periodontium; 1: bleeding upon mild probing; 2: the presence of supra- or subgingival calculus, no pocket > 3mm; 3: pockets of 4-5mm (partially occult black band of the probe); 4: pockets ≥6mm (occult black band) (B).
Stimulated saliva flow was obtained between 8:30 and 9:00a.m. To obtain the sample, the patients were instructed to chew on a piece of paraffin for 5min, at least 1h after eating and drinking. The saliva secreted in the first 2min was discarded. The saliva secreted over the next 5min was then collected in a plastic container. The saliva was collected before any intraoral intervention (examination, brushing, etc.). The samples were centrifuged at 3000rpm for 20min and then frozen at −80°C in Eppendorf tubes.
Measurement of glutathione peroxidase and glutathione reductase activityTo measure GPx activity, 120μL of saliva were incubated in a final volume of 3mL with phosphate-EDTA buffer (100mM, pH 7.5, EDTA-Na2 1mM) in the presence of 30μL of NADPH (20mM), 100μL of GSH (60mM), and 4μL (1IU) of GRd for 5min at room temperature. We then added 100μl of cumene hydroperoxide solution (36mM) and measured GPx activity after the oxidation of the NADPH for 3min at a wavelength of 340nm using a cuvette spectrophotometer.10 GRd activity was measured by a similar procedure: 35μL of sample were added to 508.5μL of an extemporaneous solution consisting of phosphate buffer (100mM, pH 7.5) and GSSG at a concentration of 2.5mM. After incubation for 5min at room temperature, we added 8.5μL of NADPH 12mM to trigger the reaction, and the oxidation of NADPH was measured for 3min at 340nm with the same cuvette spectrophotometer. In both cases, non-enzymatic oxidation of NADPH was subtracted from the total oxidation rate. The activity of both enzymes was expressed in nmol/min/mg protein.
Measurement of oxidized glutathione and reduced glutathioneTo measure GSH, 10μL of saliva were incubated with 10μL of a solution of ethanol o-phthaldehyde (OPT, 1mg/mL) and 180μL of phosphate buffer (sodium phosphate 100mM, 2.5mM EDTA-Na2, pH 8.0) for 15min at room temperature. Sample fluorescence was then read in a plate fluorometer. The quantification of GSH was carried out by comparing the fluorescence produced by the samples versus that of a standard solutions curve with known concentrations of GSH.11
To measure GSSG concentration, 25μL aliquots of saliva were incubated with 10μL of a N-ethylmaleimide solution (NEM, 5mg/mL in distilled water) for 40min at room temperature. NEM prevents the oxidation of GSH present in the sample to GSSG. After incubation, 760μL of NaOH 0.1N were added. We then added 10μL of the sample with NEM and NaOH, 10μL of OPT and 180μL of NaOH to the wells of a microplate. OPT reacts with GSSG at alkaline pH, emitting fluorescence in direct proportion to the amount of GSSG present. Incubation was carried out for 15min at room temperature, and the fluorescence was then read with a plate fluorimeter. The quantification of GSSG was carried out by comparing the fluorescence produced by the samples versus that of a standard solutions curve with known concentrations of GSSG.11 The values of GSH and GSSG were expressed in nmol/mg protein.
Statistical analysisAll the data are reported as the mean±standard deviation. Differences between the different variables were tested using a one-way analysis of variance (ANOVA), followed by a Bonferroni test to compare the differences between each of the study groups. Calculations were performed using the statistical package for the social sciences (SPSS) version 21.0 (licensed by the University of Granada). Values of p<0.05 were considered statistically significant.
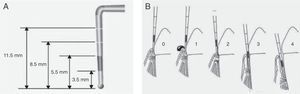
ResultsTable 1 shows the descriptive statistics and salivary concentrations of GPx, GRd, GSH and GSSG, the GSSG/GSH ratio, and the CPI in healthy subjects and patients with T2DM with good and poor metabolic control included in the study.
Descriptive statistics and parameters determined in the saliva of healthy individuals and patients with DM2.
| Control (n=19) | DM2 good metabolic control (n=24) | DM2 poor metabolic control (n=27) | |
|---|---|---|---|
| Age | 42.2±3.11 | 48.7±4.8 | 54.4±7.3 |
| Males/females | 11/8 | 11/13 | 15/12 |
| HbA1c (%) | 5.27±0.93 | 6.60±0.50** | 9.63±1.22**,*** |
| Years with diabetes | – | 8.3±1.3 | 17.1±2.8*** |
| Patients treated with insulin (%) | – | 20.8 | 62.9 |
| GPx (nmol/min/mg prot) | 26.89±4.88 | 42.12±4.38** | 16.02±2.25**,*** |
| GRd (nmol/min/mg prot) | 3.98±1.17 | 5.76±2.06** | 1.83±0.60**,*** |
| GSH (nmol/mg prot) | 51.14±3.76 | 38.14±7.88** | 41.89±6.39** |
| GSSG (nmol/mg prot) | 23.26±4.02 | 38.31±6.14** | 47.66±5.76**,* |
| GSSG/GSH ratio | 0.47±0.25 | 1.03±0.26** | 1.23±0.32**,* |
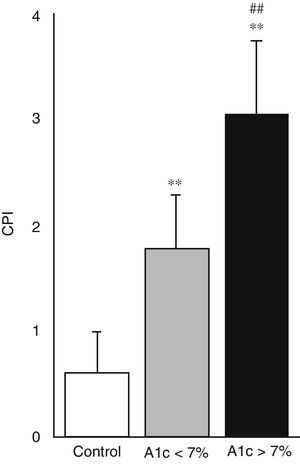
| CPI | 0.61±0.39 | 1.78±0.51** | 3.06±0.68**,*** |
Good metabolic control: HbA1c<7%.
Poor metabolic control: HbA1c>7%.
CPI: Community Periodontal Index; DM2: type 2 diabetes mellitus; GPx: glutathione peroxidase; GRd: glutathione reductase; GSH: reduced glutathione; GSSG: oxidized glutathione; HbA1c: glycosylated hemoglobin.
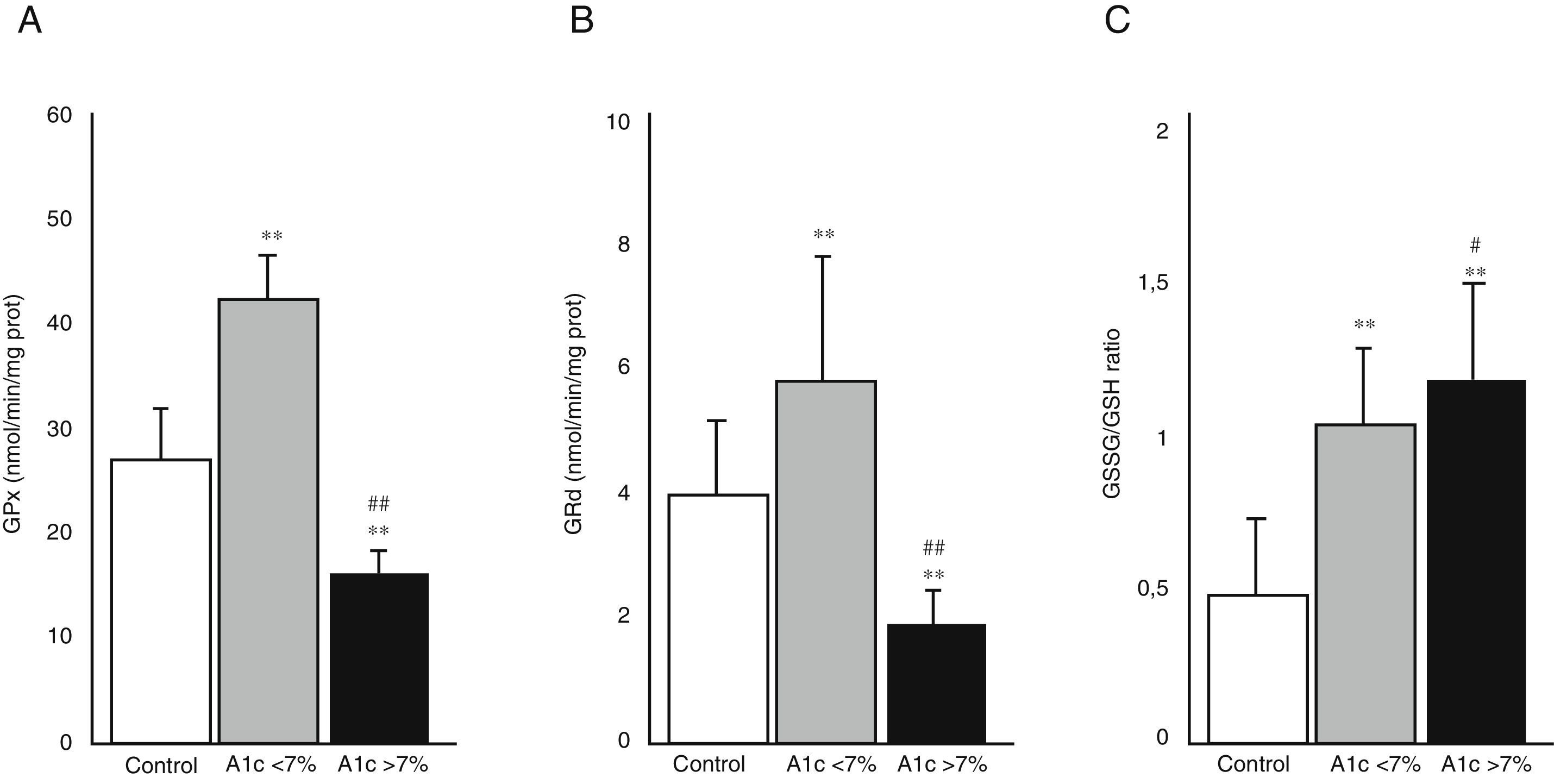
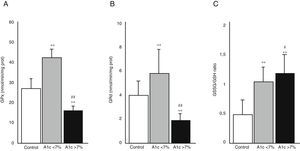
Fig. 3 shows the activity of the enzymes GPx (A) and GRd (B), as well as the GSSG/GSH ratio (C) in the three study groups. The diabetic patients with good metabolic control (HbA1c<7%) showed an increase in the activity of both enzymes compared with the control group (p<0.001). By contrast, the diabetic patients with poor metabolic control (HbA1c>7%) showed a significant decrease in GPx and GRd activity as compared to both the control group (p<0.001) and the group of diabetic patients with good metabolic control (p<0.001). Fig. 3C shows the GSSG/GSH ratio calculated from the GSSG and GSH levels recorded in the saliva samples of the patients included in the study. The ratio was found to be significantly higher in both groups of diabetic patients than in the control group (p<0.001). A significant difference was also seen between those diabetic patients with poor metabolic control and those with good metabolic control (p<0.05).
Activity of the enzymes glutathione peroxidase (GPx) (A), glutathione reductase (GRd) (B) and oxidized glutathione/reduced glutathione (GSSG/GSH) ratio (C) in the control group, patients with DM2 and good metabolic control (A1c<7%) and patients with DM2 and poor metabolic control (A1c>7%).
#p<0.05 vs A1c<7%.
**p<0.001 vs control group.
##p<0.001 vs A1c<7%.
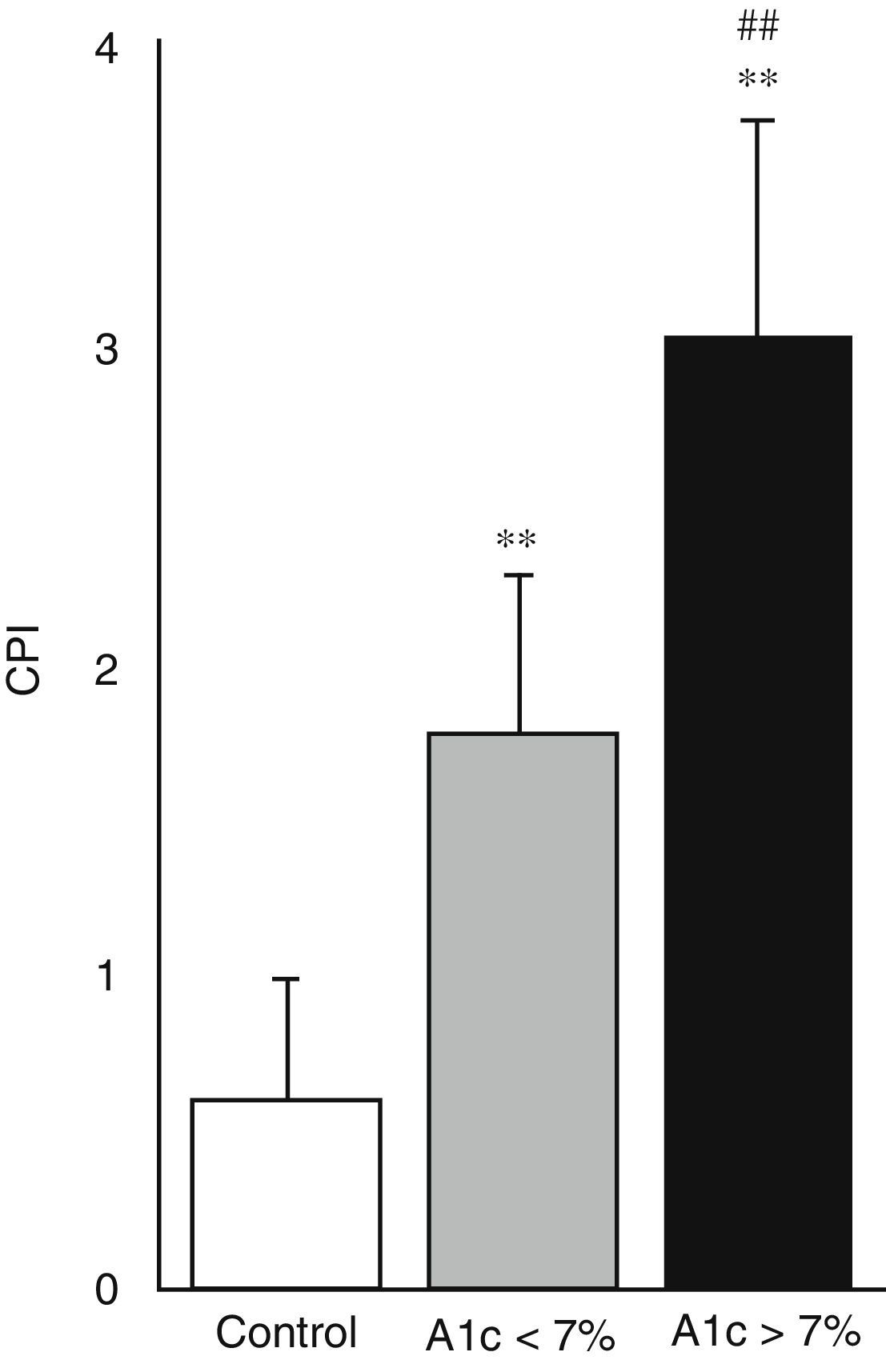
Fig. 4 shows the periodontal health status of the three study groups, based on the CPI. Both diabetic groups had a greater CPI and therefore poorer periodontal health compared with the control group (p<0.001). Likewise, the CPI was found to be greater in those diabetic patients with poor metabolic control than in those with good metabolic control (3.06±0.68 versus 1.78±0.51; p<0.001).
DiscussionMany studies have found oxidative stress to be increased in the serum of diabetic patients, secondary to an excessive production of ROS and a decrease in the host antioxidant defence mechanisms.8,12 Few studies in saliva are available, though the existing evidence suggests that similar proportional changes may exist at salivary level.13,14
Our results show that both the GSSG/GSH ratio and periodontal health, as assessed by the CPI, are significantly different in diabetic patients and controls. In the diabetic patients, both parameters were significantly greater in the individuals with poor metabolic control than in those with good metabolic control (Figs. 3C and 4). Therefore, our study shows that poorer metabolic control in patients with T2DM is associated with higher oxidative stress levels in saliva and poorer periodontal health.
Independently of the type of diabetes involved, the available evidence suggests that the increase in serum oxidative stress is associated with the presence of microangiopathic, macroangiopathic and neuropathic complications of diabetes mellitus.12 Furthermore, the increased production of ROS in diabetic individuals worsens insulin action at the peripheral level and contributes to pancreatic beta cell dysfunction.15
Very few studies have investigated whether the antioxidant capacity in the saliva of diabetic patients differs from that of healthy subjects. Belce et al. reported a decrease in sialic acid and superoxide dismutase (SOD) in unstimulated whole saliva in patients with DM1, and concluded that these lower values could explain the poorer oral hygiene of these patients.16 By contrast, other studies have described greater antioxidant activity in the saliva of patients with DM1 – the antioxidant markers being seen to increase in parallel to HbA1c.13,17 In our study, the salivary GSSG/GSH ratio was increased in diabetic patients at the expense of two factors: an increase in GSSG and a decrease in GSH levels. Despite the older age of patients with diabetes (not statistically significant), no studies assessing the independent effect of this factor upon the salivary oxidative stress markers are available. The GSSG/GSH ratio is the best cellular oxidative stress biomarker, and its increase – which was seen to be greater in the diabetic patients with poor metabolic control – could reflect greater GSH degradation in redox reactions, at the expense of a lesser synthesis.14 Reduced glutathione is an ubiquitous tripeptide that constitutes the main non-enzymatic antioxidant agent in the body, protecting the cells from free radical-induced damage. Its main function is the direct clearance of ROS and the restitution of other antioxidants such as vitamin E and ascorbic acid to their reduced forms.18 Our findings coincide with those of other studies that show that chronic hyperglycemia and oxidative stress in diabetic patients is accompanied by increased GSH oxidation – with the consequent reduction of its serum levels.19 In saliva, Gumus et al. in 2009 carried out the first case-control study discriminating between types of diabetes, in which a significant decrease was observed in the GSH levels of patients with DM1 versus individuals with DM2 and controls. This decrease did not result in a lessened salivary antioxidant capacity among the individuals with DM1.20
Another marker that should be taken into consideration in the saliva samples of our participants is the change in enzymes of the glutathione cycle: GPx and GRd (Fig. 3A and B). Both enzymes reflect the capacity of the cell to maintain the GSSG/GSH ratio and, thus, the antioxidant defensive capacity of the body. In our study, GPx and GRd activities were increased in diabetic patients with good metabolic control as compared to the control group. This increase could reflect a compensating mechanism of the body, designed to prevent oxidative damage.14 In the case of increased ROS production secondary to sustained chronic hyperglycemia, there is an increase in the activity of SOD and other antioxidant enzymes such as GPx and GRd in an attempt to clear the excessive presence of free radicals.14 On the other hand, in the diabetic patients with poor metabolic control (mean HbA1c 9.63%), a decrease was observed in the activity of both enzymes compared with both the control group and the diabetic patients with good metabolic control (p<0.001 in both cases). It is known that deficient metabolic control results in intense free radical production for a prolonged period of time, depleting the compensating capacity of the antioxidant enzyme systems and causing structural damage to the proteins exposed to advanced glycation products.21 Protein glycation not only affects hemoglobin but also proteins that participate in the host antioxidant defence systems, such as SOD, GPx and GRd, which act against ROS.22 Accordingly, poorly controlled diabetes with a glutathione system damaged by the inactivation of GPx and GRd could contribute to the development and/or progression of different complications of the disease.12,23
With regard to these complications, the main contribution of our study was the attempt to correlate this increase in salivary oxidative stress to the main oral manifestation of diabetes, periodontal disease. Fig. 4 shows that both groups of patients with DM2 exhibited a higher CPI, indicative of poorer periodontal health compared with the controls. Among the diabetic patients, those with poor metabolic control had poorer periodontal health than those with good metabolic control. Since the GSSG/GSH ratio is considered to be a reliable biomarker of cellular oxidative stress, an increase in this ratio along with a higher CPI supports the hypothesis of the participation of oxidative stress in the pathogenesis of periodontal disease in diabetic patients. Many studies support this idea and coincide with our findings.8,20 In patients with periodontal disease, polymorphonuclear neutrophils (PMN) have been shown to be functionally activated and to produce large amounts of superoxide anion (O2−) and other ROS. Similarly, an increased oxidative response of peripheral PMNs has been seen in patients with juvenile and adult periodontitis. This increase in oxidative response in turn has been related to the periodontal condition of the patients.8,24
Based on our results, it may be concluded that poorer metabolic control in patients with T2DM leads to greater levels of salivary oxidative stress, which in turn results in poorer periodontal health. As regards its clinical relevance, this study represents an attempt to show the value of the measurement in saliva of certain markers of the glutathione cycle (GSSG/GSH, GPx, GRd) as possible predictors for the development and/or the progression of periodontal disease in patients with T2DM. The limitations of the study include its small sample size, which precluded the selection of a control group of patients without diabetes and with periodontal disease. Another possible limitation is the great variability found in the literature regarding the diabetic populations studied, the parameters measured, and the methods used. This means a lack of reference protocols or standards for determining salivary oxidative stress. Nor does it make it easier for us to compare our results with others. Further studies are therefore needed in this field. An additional interesting topic for future research would be the way in which antioxidant agents may influence the periodontal changes in patients with diabetes.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Arana C, Moreno-Fernández AM, Gómez-Moreno G, Morales-Portillo C, Serrano-Olmedo I, de la Cuesta Mayor MC, et al. Incremento de los parámetros de estrés oxidativo salival en pacientes con diabetes tipo 2: relación con la enfermedad periodontal. Endocrinol Diabetes Nutr. 2017;64:258–264.