Aggressive pituitary tumors (APT) are characterized by large tumor size, invasion of adjacent structures, little response to conventional treatment, high rate of recurrence, and elevated morbidity.1–3 The prevalence of APT has been estimated in less than 10% of pituitary adenomas (PA). Corticotropinomas are the most prevalent (45%) followed by prolactinomas (30%).3 We here report a clinical case showing the long-term successful therapeutic outcome to multimodal therapy in a life-threatening aggressive prolactinoma.
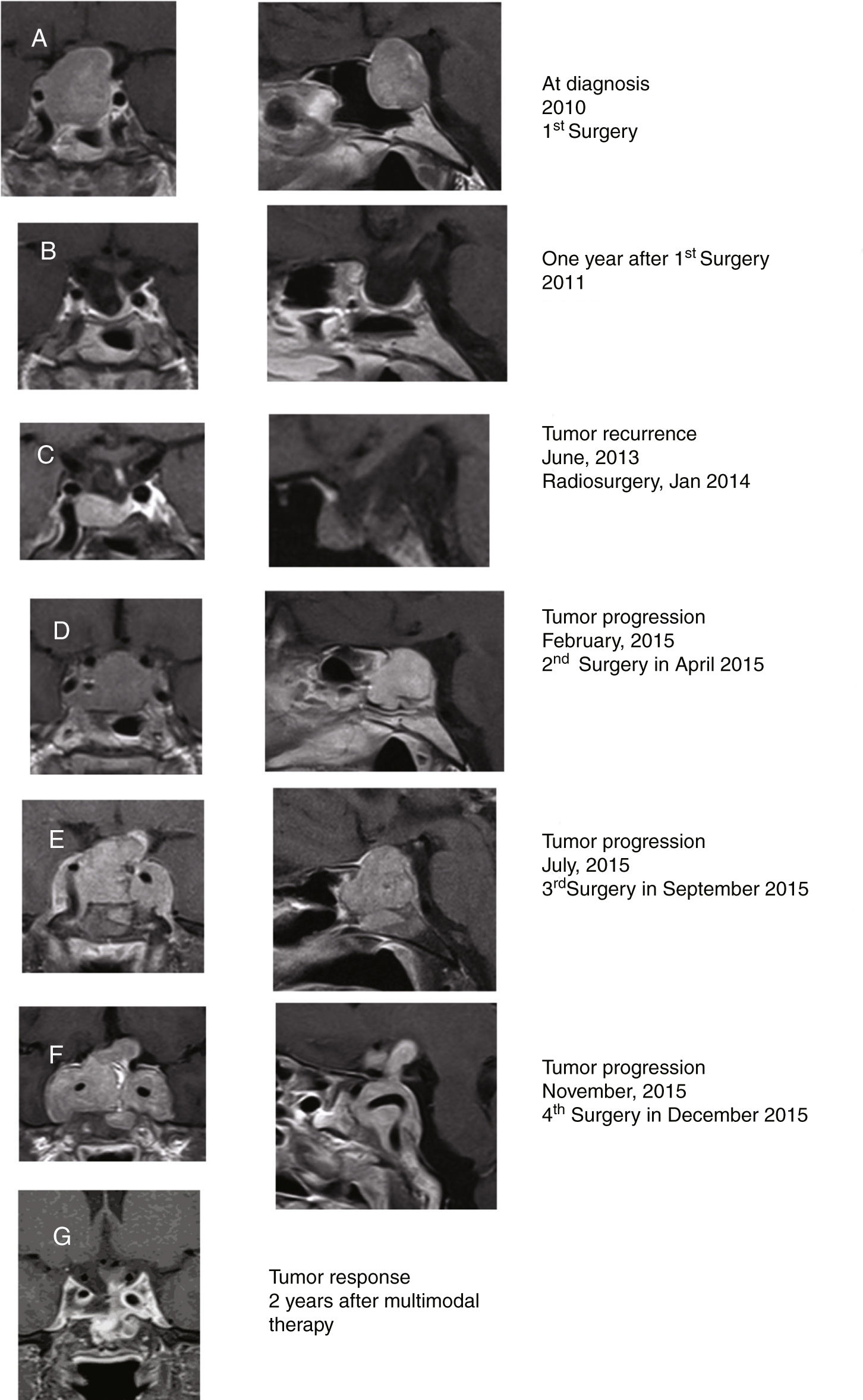
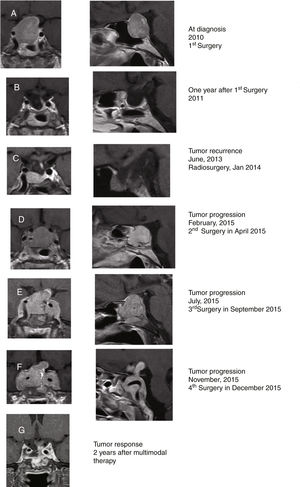
A 48-year-old woman consulted in other center in 2010 for diminishing visual acuity progressively in both eyes with bitemporal hemianopia and galactorrhea for 3 months. She was diagnosed with macroprolactinoma (PRL 505ng/ml; normal IGF-1; and pituitary macroadenoma, 25×24×20mm; Fig. 1A). The patient initially underwent surgery in 2010 through endonasal endoscopic approach (EEA) with an almost complete resection of the tumor (histopathological study compatible with PA with diffuse expression for PRL and focal for GH; serum PRL 6ng/ml; Fig. 1B). In June 2013, pituitary MRI showed an intrasellar tumor nodule (7×11×8mm) compatible with PA recurrence (Fig. 1C). Cabergoline dose was increased from 0.5mg twice a week to 0.5mg/day without clinical response. She was treated with radiosurgery (total dose, 20Gy) in January 2014. In April 2015, it was decided to reoperate due to the continuous radiological tumor progression, performing a subtotal resection of the tumor (Fig. 1D). In July 2015, after suffering a clinical progression (deterioration of visual acuity with ophthalmoparesis), a rapid growth of the lesion was observed, being reoperated again via EEA (Fig. 1E). The histopathological study was compatible with PA with immunostaining for prolactin (densely granulated subtype) and a 10% Ki67 cell proliferation index.
Coronal (left) and sagittal (right) gadolinium-enhanced T1-weighted MRI images at diagnosis (A). MRI images one year after surgery showing postoperative changes in relation to almost complete resection of the tumor, with the optic chiasm in normal situation and slight displacement of the pituitary stalk to the left (B). MRI in 2013 showing a radiological tumor progression that was treated with radiosurgery in 2014 (C). MRI in February 2015 showing radiological tumor progression which was treated with a subtotal resection of the tumor (D). MRI in July 2015 showing radiological tumor progression which was reoperated via an endonasal endoscopic posterior-transphenoidal approach with a subtotal resection of the tumor (E). MRI in November 2015 showing radiological tumor progression (F). Tumor response after 2 years (G) of multimodal therapy with radiotherapy, DA, SSA, and TMZ showing a significant reduction in tumor size.
The patient was seen for the first time in our center in December 2015. She presented with complete bilateral blindness rapidly developed in the last 48h, low level of consciousness, and severe headache not controlled even with morphine. She was under treatment with hydrocortisone, 30mg/day, levothyroxine 100μg/day and cabergoline 0.5mg/day. Pituitary MRI showed a voluminous sellar and suprasellar lesion (43×52×40mm) with invasion of both cavernous sinuses and engulfment of both carotid arteries. The lesion widely destroyed the sella turcica, the quadrilateral lamina and grows behind the clivus, findings compatible with a histologically aggressive pituitary tumor (Fig. 1F). An indium-111 pentetreotide scan (Octreoscan) showed a lesion located in the suprasellar region with extension to the cavernous sinus and the clivus with intense activity. A clinical assessment by a multidisciplinary team recommended completing treatment with palliative surgery, dopamine agonists (DA), somatostatin analogs (SSA), temozolomide (TMZ), and postoperative radiotherapy one month after surgery.
She was reoperated for the fourth time on December 2015, by subtotal tumor resection (∼50%) through EEA focused on decompressing the optic nerves. Histopathological study showed a densely granulated prolactinoma with histological signs of aggressiveness (cellular pleomorphism and nuclear atypia, 2 mitotic figures for 10 high power fields, bone infiltration and Ki67 10%). On this occasion the immunostaining for GH was negative. Methylation status of the O-6-methylguanine-DNA methyltransferase (MGMT) promoter by methylation-specific PCR with the EpiTect Bisulfite kit (QIAGEN) was negative. After surgery, the patient regained vision in the left eye. At discharge, she continued treatment with hydrocortisone, 30mg/day, levothyroxine 100μg/day, and cabergoline 0.5mg/day. Lastly, she was treated with stereotactic radiotherapy (57.1Gy), and started therapy with SSA (lanreotide autogel 120mg/28 days subcutaneously) and received adjuvant TMZ (75mg/m2; 120mg/day) for 3 weeks, followed by TMZ cycles every 28 days (120mg/m2 oral/24h for 5 days; ending in March 2018 with a total of 28 cycles).
Two years after last surgery the patient was well, although she continued with amaurosis of the right eye and hypopituitarism, but with adequate visual acuity in the left eye. She did not have headaches and led an almost normal life. The last pituitary MRI performed in January 2018 showed postoperative changes, with a partially empty sella with descent of the optic chiasm, without signs of tumor recurrence (Fig. 1G).
The clinical case herein reported highlights the long-term therapeutic success of multimodal therapy in a patient with a life threatening aggressive prolactinoma.
The first-line treatment of prolactinoma, regardless of size, is medical therapy with DA, preferably cabergoline. This treatment achieves a normalization of prolactin levels, a reduction in tumor size and a restoration of gonadal function in a large number of patients.4 Some prolactinomas are resistant to DA (inability to normalize prolactin levels and to decrease tumor size ≥50%).5
The patient herein reported showed a late tumor relapse 3 years after the first successful surgery with a consequent rapid tumor growth despite treatment with high doses of cabergoline and without response to radiosurgery. Histopathological study was compatible with aggressive prolactinoma.1
Although infrequent, some PA recur despite medical and/or surgical therapy ± adjuvant radiotherapy. In these cases, it would be appropriate to consider a new surgery with or without a second course of radiotherapy in order to control tumor growth. In the present case, the earlier use of fractionated stereotactic radiotherapy associated with TMZ would probably have reduced the number of reoperations. The experience with re-irradiation in recurrent PA is very limited since only 11% of patients are re-irradiated. An increased long-term risk of developing hypothyroidism, optic neuropathy, stroke, neurocognitive dysfunction, second brain tumors, and temporal lobe necrosis should be taken into account in re-irradiated patients. At present, the real therapeutic effect of re-irradiation on aggressive prolactinomas is unknown, due to the small number of patients reported so far.6
Therapy with SSA has been proposed as an alternative in prolactinomas resistant to DA, since those prolactinomas express somatostatin receptors and some studies have shown that SSA suppress in vitro PRL release in human PA.7,8 On the other hand, the antiproliferative effect in neuroendocrine tumors expressing SS receptors is also well known.9 The simultaneous treatment with SSA together with radiotherapy and TMZ, does not allow us to know the therapeutic effect of SSA separately in this case.
The tumor response rate to TMZ, as well as its relationship with the MGMT status and the Ki-67% cell proliferation index in APT in aggressive prolactinomas have been recently reviewed.10 The tumor response (complete or partial) rate in these tumors treated with TMZ was 53.6%; whereas 12.5% showed stable disease. MGMT staining has been proposed as a negative strong predictor to the outcome of therapy with TMZ.
In conclusion, this clinical case shows how multimodal therapy is able to achieve an adequate long-term tumor response with adequate tolerance in life-threatening aggressive prolactinoma. It is probable that multimodal therapy increases the therapeutic response rate in these patients compared to isolated adjuvant treatment using re-irradiation, DA, SSA or TMZ, separately.
Conflict of interestThe authors have no conflict of interest.