Bariatric surgery (BS) leads to several changes in nutritional habits that can be attributed to different mechanisms. Some of these changes could be achievable with a preoperative nutritional intervention. The objective was to evaluate dietary modifications during the preoperative and postoperative periods of BS.
MethodsProspective observational study of patients who underwent BS between 2010 and 2014 at the Hospital del Mar; 60 consecutive patients were included. Food consumption was measured by a validated food-frequency questionnaire at inclusion in the bariatric surgery program, after preoperative nutritional intervention, and one year after surgery. Generalized estimating equation models were used to assess differences in food group intake during follow up.
ResultsEnergy intake significantly decreased from inclusion to 1 year of surgery (p=0.003). After the preoperative intervention and prior to surgery, there was an increase in intake of nuts, vegetables, poultry and rabbit, fruit, fish and skimmed milk products and a decrease in bread, soft drinks and pastry. At one year post-surgery, a continued decrease in the consumption of bread and soft drinks and an increase in nuts was observed (linear non-quadratic trend). Consumption of fruit, pastry, fish and skimmed milk products remained stable showing a linear and quadratic trend. Vegetables and poultry and rabbit increased in the preoperative period and decreased after surgery, showing a quadratic but not linear trend.
Conclusionsa preoperative nutritional intervention in morbidly obese patients can associate favorable dietary changes that are mostly maintained one year after bariatric surgery.
La cirugía bariátrica produce diferentes cambios en los hábitos alimentarios que se han atribuido a diferentes mecanismos. Algunos de estos cambios se podrían conseguir con una intervención nutricional preoperatoria. El objetivo fue estudiar los cambios dietéticos durante el periodo preoperatorio y postoperatorio de la cirugía bariátrica.
MétodosEstudio prospectivo de pacientes intervenidos de cirugía bariátrica entre los años 2010 y 2014 en el Hospital del Mar de Barcelona. Se incluyeron 60 pacientes consecutivos. El registro de alimentos se midió mediante cuestionarios de frecuencia de consumo de alimentos a la inclusión en el programa de cirugía bariátrica, después de una intervención nutricional preoperatoria y un año después de la cirugía. Se usaron las ecuaciones de estimación generalizadas para determinar diferencias en el consumo de los grupos de alimentos durante el seguimiento.
ResultadosEl consumo de energía disminuyó desde la inclusión un año después de la intervención (p=0,003). En el periodo preoperatorio y antes de la cirugía se detectó un aumento en el consumo de frutos secos, vegetales, aves y conejo, fruta, pescado y lácticos desnatados y un descenso en el consumo de pan, bebidas azucaradas y bollería. Un año después de la cirugía, se observó que el consumo de pan y bebidas azucaradas siguió descendiendo y el de frutos secos siguió aumentando (tendencia lineal pero no cuadrática). El consumo de fruta, pescado y lácticos desnatados se mantuvo estable (tendencia lineal y cuadrática). El consumo de vegetales, aves y conejo aumentó en el periodo preoperatorio y disminuyó después de la cirugía (tendencia cuadrática pero no lineal).
ConclusionesUna intervención nutricional preoperatoria en pacientes con obesidad mórbida puede asociarse a cambios dietéticos favorables, los cuales se mantienen en su mayoría un año después de la intervención.
Bariatric surgery (BS) is an effective therapeutic strategy to achieve significant and sustained weight loss, with beneficial effects on co-morbidities associated with obesity.1 Most weight loss in BS patients is achieved in the first year after surgery, followed by slight weight regain in the following years.2 BS can produce weight loss by several mechanisms such as hormonal or microbiota changes.3 Nevertheless, the most important mechanism is the dramatic drop in energy intake in the early months after surgery. During the first three months, diet consists of liquids and soft solids, from three months to one year patients present several food intolerances that require nutritional adaptations and, one year after the procedure, diet should be solid, varied and hypocaloric.4–8
Nutritional support is mandatory to accomplish these changes dietary habits9 and provide alimentary reeducation to achieve permanent changes in healthy eating habits. The final aim of this intervention is for patients to continue losing weight and maintain the attained weight loss in the long-term. Moreover, other mechanisms such as variations in taste or dumping syndrome can also influence postoperative dietary habits.10,11 In this respect, it is difficult to ascertain which variations in dietary consumption patterns are caused by alimentary reeducation and which are mediated by other mechanisms directly or indirectly related to the surgical procedure.
On the other hand, prior to BS, patients have a high energy intake and unhealthy dietary habits such as snacking between meals or eating only two or three times per day.12 Updated clinical guidelines recommend that nutritional intervention should start before the surgical procedure9 since preoperative weight loss could be beneficial in reducing surgical complications.13,14 Nevertheless, studies evaluating dietary changes after BS did not consider the potential impact of this pre-surgical nutritional intervention.5–7,15–17 Assuming that this intervention associates changes in dietary behavior with inducing weight loss, the dietary habits of patients achieved after the preoperative nutritional intervention may bear some similarities to those already achieved one year after the procedure. Moreover, a comparison of dietary patterns after the preoperative intervention with those one year post-bariatric surgery could determine which changes in dietary habits are associated mainly with mechanisms related to the surgical procedure.
Thus, the aim of the present study was to evaluate dietary modifications during the preoperative and postoperative periods of BS.
MethodsA non-randomized prospective cohort study was conducted in consecutive severely obese patients undergoing BS at the Hospital del Mar, Barcelona. Patients were aged between 18 and 55 years and met the 1991 BS criteria of the National Institutes of Health.18 Patients lost during follow-up or those who did not complete all the food-frequency questionnaires (FFQ) were excluded (reviewer 2, comment 3). The Ethics Committee of our Institution (Parc de Salut Mar) approved the protocol. All research was performed in accordance with ethical guidelines of the 1975 Declaration of Helsinki. All patients gave their written informed consent before entering the study.
All patients were evaluated at the time of inclusion in the BS program (before receiving any dietary intervention), preoperatively and at 3, 6, and 12 months post-surgery. Protocol appointments included anthropometric measurements. Individuals wore underwear. BMI was determined as weight divided by height squared (kg/m2). The percentage of excess weight loss (%EWL) was based on weight in excess of that corresponding to a BMI of 25kg/m2 for each patient.
Food and nutrient intake were measured with a validated food-frequency questionnaire (FFQ),19 administered by a trained nutritionist at inclusion, preoperatively and at 12 months post-surgery. The FFQ comprised 165 items, including foodstuffs, alcohol, and non-alcoholic beverages. For each food item, participants were asked their usual consumption for the nine frequency categories, ranging from never or less than once per month to six or more times per day. The results of all FFQ were analyzed at study completion. Therefore, individual FFQs results were not used to improve dietary habits.
Measurements of smoking habit and demographic and socioeconomic variables were obtained through structured standard questionnaires administered by trained personnel. Participants were categorized as non-smokers, former smokers (less than 1 year of smoking cessation) and current smokers (at least 1 cigarette/day on average in the last year). Maximum education level attained was elicited and recorded for analysis as primary school, secondary school or further education.
All patients included in the study followed the standard nutritional intervention included in the BS program of the Hospital del Mar. The intervention was performed by a nutritionist with the support of a psychologist. The preoperative intervention includes 6 monthly group sessions focused on achieving changes in dietary habits to facilitate adaptation after surgery. Patients had to include new foods items, remove others from their diet and reduce size of the portions. Education on the choice of foodstuffs and meal planning was also encouraged. Ten days before surgery a very low-calorie diet was prescribed to achieve additional weight loss. Preoperative weight and food consumption were recorded just before this very low-calorie diet. In the immediate postoperative period, nutritional support appointments were scheduled at 2, 4 and 8 weeks with the aim of patients progressing from a liquid diet to a soft solid diet. Thereafter, appointments were held at 6 and 12 months to detect and manage possible dietary intolerances. One year post-surgery diet should be solid, varied and low-calorie.
Indication for the type of surgical procedure (sleeve gastrectomy and gastric bypass) was based on clinical criteria and the consensus of the BS Unit.
Categorical variables were expressed as percentages and continuous variables as mean and standard deviation or the median and interquartile range. Variables of food groups and nutrients are described as mean and its 95% confidence interval. Generalized estimating equation models were used to assess differences in food group and nutrients intake during follow up, taking into account repeated measurements within each participant and adjusting for age, sex and educational level). Statistical significance for linear and quadratic trends was obtained. For all the analyses, a p value<0.05 was considered statistically significant and were conducted using SPSS for Windows (version 20.0) statistical software package (SPSS Inc., Chicago, Ill., USA).
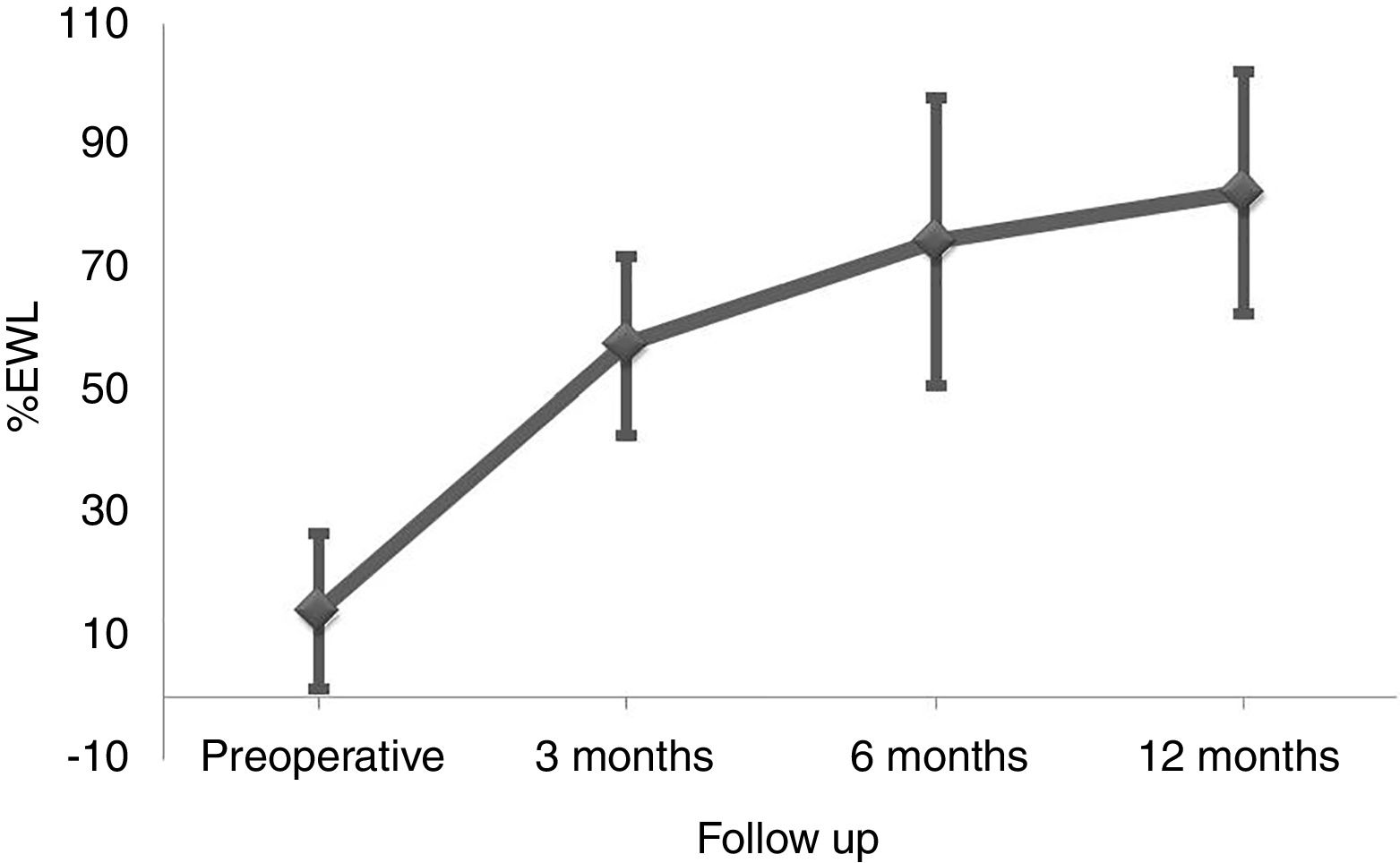
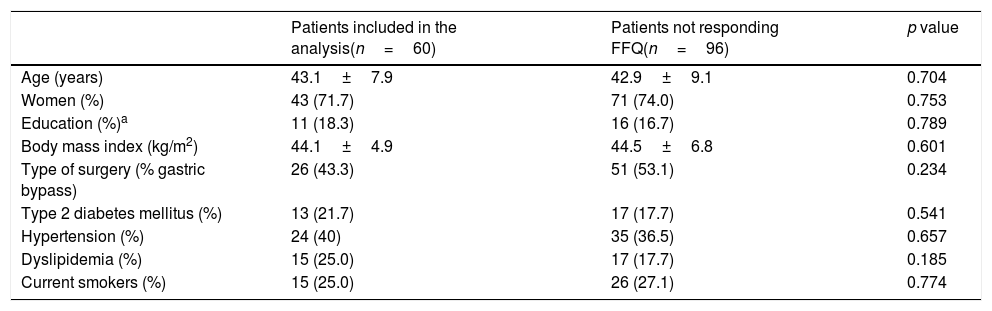
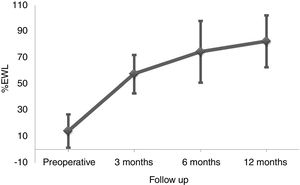
ResultsBetween March 2010 and October 2014, 164 patients underwent BS at the Hospital del Mar in Barcelona. Of these, 8 patients were lost to follow-up and 96 did not complete at least one of the three FFQ (13 did not answer the preoperative FFQ, 75 did not answer the 12 months after surgery FFQ and 8 did not answer neither of them). Thus, 60 patients were included in the study and completed the follow-up period. No differences in the clinical characteristics between the included subjects and those who did not answer he FFQ were detected (Table 1). Preoperative %EWL was 14.2±12.7 and percentage total body weight loss 4.7±4.8. As shown in Fig. 1 weight loss after the surgery was progressive, reaching a %EWL of 82.5±23.5 and percentage total body weight loss of 33.5±7.2 at 12 months. Patients who did not answer the FFQ presented a similar %EWL in comparison to the included patients, both in the preoperative evaluation (11.4±9.8, p=0.408) and 12 months after BS (80.1±21.2, p=0.504).
Baseline characteristics of severe obese patients undergoing bariatric surgery.
| Patients included in the analysis(n=60) | Patients not responding FFQ(n=96) | p value | |
|---|---|---|---|
| Age (years) | 43.1±7.9 | 42.9±9.1 | 0.704 |
| Women (%) | 43 (71.7) | 71 (74.0) | 0.753 |
| Education (%)a | 11 (18.3) | 16 (16.7) | 0.789 |
| Body mass index (kg/m2) | 44.1±4.9 | 44.5±6.8 | 0.601 |
| Type of surgery (% gastric bypass) | 26 (43.3) | 51 (53.1) | 0.234 |
| Type 2 diabetes mellitus (%) | 13 (21.7) | 17 (17.7) | 0.541 |
| Hypertension (%) | 24 (40) | 35 (36.5) | 0.657 |
| Dyslipidemia (%) | 15 (25.0) | 17 (17.7) | 0.185 |
| Current smokers (%) | 15 (25.0) | 26 (27.1) | 0.774 |
FFQ; food frequency questionnaires.
Median energy intake was 2765kcal/day (interquartile range: 2450–3079) at inclusion in the BS program. It decreased in the preoperative evaluation to 2215kcal/day (1999–2430) and then it remained stable 1 year after surgery [2353kcal/day (2108–2598)], showing a significant linear (p=0.003) and quadratic trend (p=0.005).
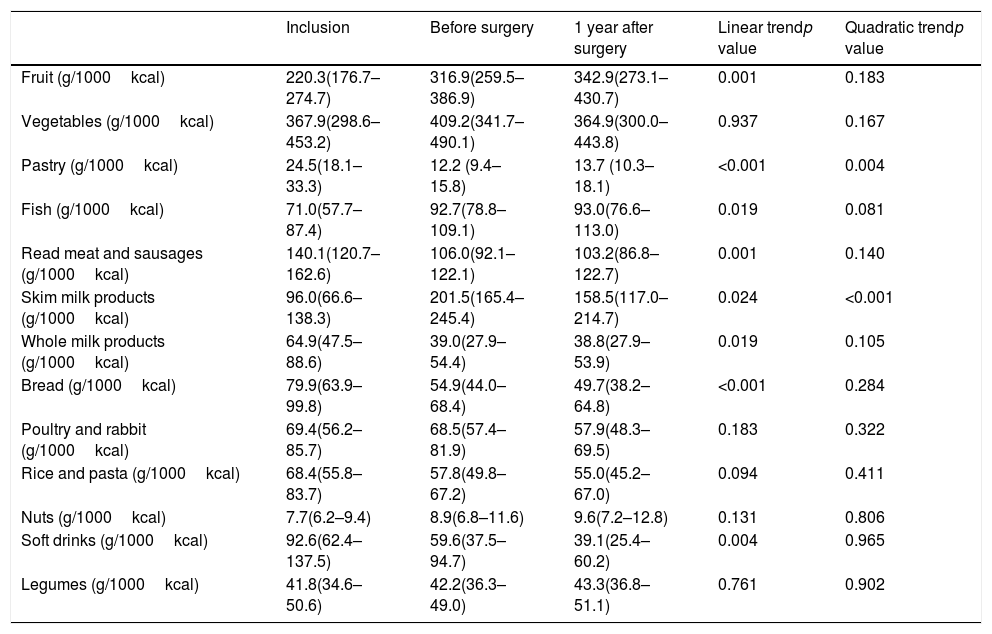
Several qualitative changes in food consumption during follow-up were observed (Table 2). After the preoperative intervention and before surgery, the intake of fruit, fish and skimmed milk products increased while a decrease in bread, red meat, whole milk products, soft drinks and pastry intake was observed. Changes in food consumption in the first year after surgery were variable. A continuous change from baseline showing a linear non-quadratic trend was observed in the consumption of bread, red meat, whole milk products and soft drinks (with a decreasing tendency). Moreover, fruit and fish presented an increasing tendency. Consumption of other nutritional groups, such as pastry and skimmed milk products remained stable with a linear and quadratic trend.
Consume of food groups in bariatric surgery patients during follow-up.
| Inclusion | Before surgery | 1 year after surgery | Linear trendp value | Quadratic trendp value | |
|---|---|---|---|---|---|
| Fruit (g/1000kcal) | 220.3(176.7–274.7) | 316.9(259.5–386.9) | 342.9(273.1–430.7) | 0.001 | 0.183 |
| Vegetables (g/1000kcal) | 367.9(298.6–453.2) | 409.2(341.7–490.1) | 364.9(300.0–443.8) | 0.937 | 0.167 |
| Pastry (g/1000kcal) | 24.5(18.1–33.3) | 12.2 (9.4–15.8) | 13.7 (10.3–18.1) | <0.001 | 0.004 |
| Fish (g/1000kcal) | 71.0(57.7–87.4) | 92.7(78.8– 109.1) | 93.0(76.6–113.0) | 0.019 | 0.081 |
| Read meat and sausages (g/1000kcal) | 140.1(120.7–162.6) | 106.0(92.1–122.1) | 103.2(86.8–122.7) | 0.001 | 0.140 |
| Skim milk products (g/1000kcal) | 96.0(66.6–138.3) | 201.5(165.4–245.4) | 158.5(117.0–214.7) | 0.024 | <0.001 |
| Whole milk products (g/1000kcal) | 64.9(47.5–88.6) | 39.0(27.9–54.4) | 38.8(27.9–53.9) | 0.019 | 0.105 |
| Bread (g/1000kcal) | 79.9(63.9–99.8) | 54.9(44.0–68.4) | 49.7(38.2–64.8) | <0.001 | 0.284 |
| Poultry and rabbit (g/1000kcal) | 69.4(56.2–85.7) | 68.5(57.4–81.9) | 57.9(48.3–69.5) | 0.183 | 0.322 |
| Rice and pasta (g/1000kcal) | 68.4(55.8–83.7) | 57.8(49.8–67.2) | 55.0(45.2–67.0) | 0.094 | 0.411 |
| Nuts (g/1000kcal) | 7.7(6.2–9.4) | 8.9(6.8–11.6) | 9.6(7.2–12.8) | 0.131 | 0.806 |
| Soft drinks (g/1000kcal) | 92.6(62.4–137.5) | 59.6(37.5–94.7) | 39.1(25.4–60.2) | 0.004 | 0.965 |
| Legumes (g/1000kcal) | 41.8(34.6–50.6) | 42.2(36.3–49.0) | 43.3(36.8–51.1) | 0.761 | 0.902 |
Data of food groups are given in mean and 95% confidential interval. Generalized estimating equation models adjusted for sex, age and educational level were used to assess differences during follow up.
A positive correlation between the decrease in caloric intake in the preoperative and the postoperative period (r=0.291, p=0.024) was observed. Despite this, a relationship between the change in caloric intake before surgery and weight loss during the first year after the surgical procedure was not detected (r=−0.054, p=0.684).
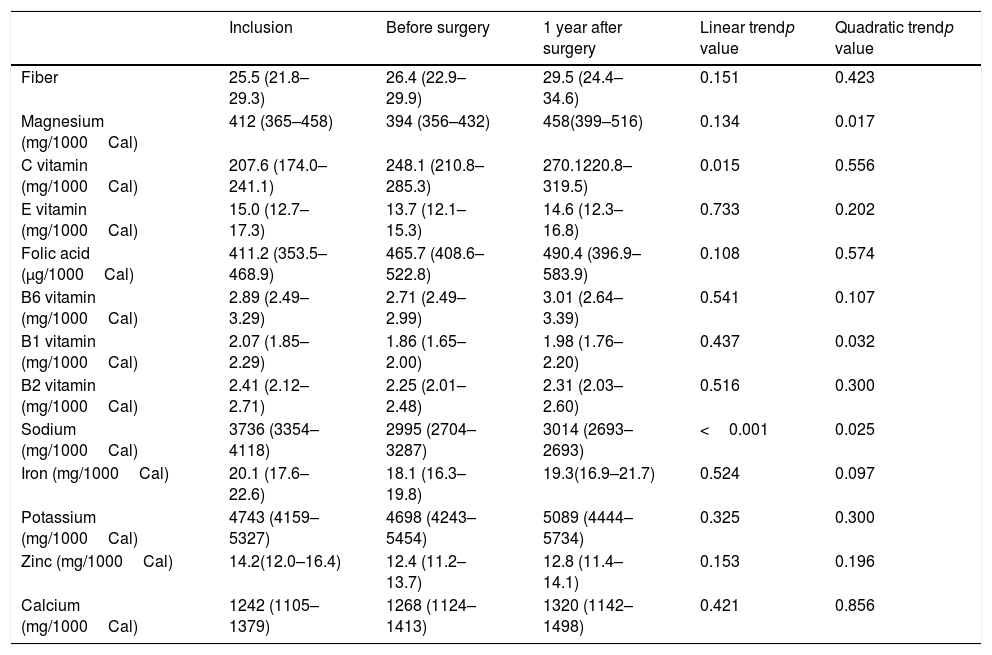
The consumption of most nutrients remained stable with the exception of vitamin C that gradually increased, Magnesium that increased from the preoperative period to 1 year after surgery and Vitamin B1 that decreased in the preoperative period and then remained stable (Table 3). The percentage of patients that reached the nutrient recommended intake according to Spanish population recommendations is shown in Table 420.
Consume of nutrients during follow-up.
| Inclusion | Before surgery | 1 year after surgery | Linear trendp value | Quadratic trendp value | |
|---|---|---|---|---|---|
| Fiber | 25.5 (21.8–29.3) | 26.4 (22.9–29.9) | 29.5 (24.4–34.6) | 0.151 | 0.423 |
| Magnesium (mg/1000Cal) | 412 (365–458) | 394 (356–432) | 458(399–516) | 0.134 | 0.017 |
| C vitamin (mg/1000Cal) | 207.6 (174.0–241.1) | 248.1 (210.8–285.3) | 270.1220.8–319.5) | 0.015 | 0.556 |
| E vitamin (mg/1000Cal) | 15.0 (12.7–17.3) | 13.7 (12.1–15.3) | 14.6 (12.3–16.8) | 0.733 | 0.202 |
| Folic acid (μg/1000Cal) | 411.2 (353.5–468.9) | 465.7 (408.6–522.8) | 490.4 (396.9–583.9) | 0.108 | 0.574 |
| B6 vitamin (mg/1000Cal) | 2.89 (2.49–3.29) | 2.71 (2.49–2.99) | 3.01 (2.64–3.39) | 0.541 | 0.107 |
| B1 vitamin (mg/1000Cal) | 2.07 (1.85–2.29) | 1.86 (1.65–2.00) | 1.98 (1.76–2.20) | 0.437 | 0.032 |
| B2 vitamin (mg/1000Cal) | 2.41 (2.12–2.71) | 2.25 (2.01–2.48) | 2.31 (2.03–2.60) | 0.516 | 0.300 |
| Sodium (mg/1000Cal) | 3736 (3354–4118) | 2995 (2704–3287) | 3014 (2693–2693) | <0.001 | 0.025 |
| Iron (mg/1000Cal) | 20.1 (17.6–22.6) | 18.1 (16.3–19.8) | 19.3(16.9–21.7) | 0.524 | 0.097 |
| Potassium (mg/1000Cal) | 4743 (4159–5327) | 4698 (4243–5454) | 5089 (4444–5734) | 0.325 | 0.300 |
| Zinc (mg/1000Cal) | 14.2(12.0–16.4) | 12.4 (11.2– 13.7) | 12.8 (11.4–14.1) | 0.153 | 0.196 |
| Calcium (mg/1000Cal) | 1242 (1105–1379) | 1268 (1124–1413) | 1320 (1142–1498) | 0.421 | 0.856 |
Data of nutrients are given in mean and 95% confidential interval. Generalized estimating equation models adjusted for sex, age and educational level were used to assess differences during follow up.
Percentage of patients that reach nutrient recommended intake according to Spanish population recomendations.20
| Inclusion | Before surgery | 1 year after surgery | p value | |
|---|---|---|---|---|
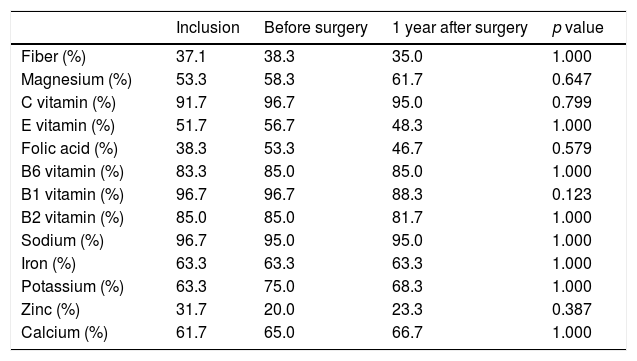
| Fiber (%) | 37.1 | 38.3 | 35.0 | 1.000 |
| Magnesium (%) | 53.3 | 58.3 | 61.7 | 0.647 |
| C vitamin (%) | 91.7 | 96.7 | 95.0 | 0.799 |
| E vitamin (%) | 51.7 | 56.7 | 48.3 | 1.000 |
| Folic acid (%) | 38.3 | 53.3 | 46.7 | 0.579 |
| B6 vitamin (%) | 83.3 | 85.0 | 85.0 | 1.000 |
| B1 vitamin (%) | 96.7 | 96.7 | 88.3 | 0.123 |
| B2 vitamin (%) | 85.0 | 85.0 | 81.7 | 1.000 |
| Sodium (%) | 96.7 | 95.0 | 95.0 | 1.000 |
| Iron (%) | 63.3 | 63.3 | 63.3 | 1.000 |
| Potassium (%) | 63.3 | 75.0 | 68.3 | 1.000 |
| Zinc (%) | 31.7 | 20.0 | 23.3 | 0.387 |
| Calcium (%) | 61.7 | 65.0 | 66.7 | 1.000 |
Generalized estimating equation models adjusted for sex, age and educational level were used to assess differences during follow up.
The present study is the first to describe nutritional changes with a nutritional intervention prior to BS. This recommendation stimulates favorable qualitative nutritional modifications with a drop in the daily energy intake. One year after surgery, these newly acquired healthy dietary habits had been largely maintained, although some of them continued to improve.
Several nutritional changes after BS16,21 are attributed to different mechanisms, some of which are a consequence of de gastrointestinal surgery, such as volume restriction, food intolerance, changes in taste or dumping syndrome.22 The most significant result of the present study was that the majority of changes in dietary habit observed one year after surgery had been present before the procedure, after completion of the preoperative nutritional intervention. A change toward healthier eating habits with a higher consumption of fruits, poultry and rabbit, fish and skimmed milk products and lower intake of pastry, bread, red meat, whole milk products and soft drinks was observed. In this respect, Ullrich et al. found a rise in healthy food consumption (poultry, fish and vegetables) and a drop in unhealthy foods (chocolate and soft drinks) in 44 patients undergoing gastric bypass. They also detected an improvement in hedonic hunger (increased drive to eat highly-palatable foods) and hypothesized that this mechanism could be determinant.21
Nevertheless, not all nutritional changes were related to the pre-surgery nutritional intervention since the consumption of some food groups continued to improve during the postoperative period: such as the decrease in bread, whole milk products, red meat and soft drinks and the increase in fruits and fish consumption. The progressive decline in red meat consumption could be attributed to the appearance of certain intolerances after BS. Hence, this is the most common intolerance described after surgery, as has been previously described in more than 50% of patients23. On the other hand, a dramatic fall in the consumption of soft drinks has been reported by other studies16,21,24 and could be attributed to the dumping syndrome, produced by accelerated gastric emptying that causes vasovagal symptoms following simple carbohydrate ingestion.11 Patients with this syndrome should avoid foods with a high glycemic index. Moreover, this decrease in consumption of sugars could be attributed to the changes in taste perception also reported after BS.10,21
Results of the present study differ from the previous one in two main aspects. First, preceding studies observed that energy intake one year after BS was around 1000–1300kcal/day4–8 contrasting with the near 2300kcal/day in this study. This finding can be explained by the fact that previous studies used dietary recalls and not FFQ to measure dietary intake. Energy intake estimation seems to be considerably lower in food recalls compared with a FFQ. Secondly, we detected that consumption of the majority of micronutrient remained stable during follow-up. However, worsening of several micronutrients was described in other studies.8,16 Regardless of this, it must be highlighted that the percentage of patients under the nutrient following the recommended intake is around 25–50% for the majority of micronutrients and that patients can present some micronutrient malabsorption with BS.25 This reinforces the recommendation of multivitamin supplementation for all the patients after BS9.
The results of the present study emphasize the importance of the nutritional intervention before BS. Moreover, preoperative weight loss is highly recommended for certain reasons, the most significant being a reduction in liver volume that can improve technical aspects of surgery and prevent surgical complications.13,14 Moreover, preoperative weight loss can have a positive impact on long-term weight loss. Thus, hypothetically, patients who incorporate more changes into their nutritional habits before and during the early months post-surgery could regain less weight when the digestive tract is adapted and a higher amount of food is tolerated. This hypothesis is supported by the findings of Moize et al., the authors found in a cohort of 355 patients who underwent a sleeve gastrectomy or gastric bypass with a 5-year-follow up and reported that preoperative energy intake was one of long term weight loss predictors.8 Despite this, other studies reported no benefits of preoperative weight loss on short and mid-term postoperative weight loss.26,27
This study is not without limitations. First, the percentage of patients who agreed to answer the FFQ was low. However, basal characteristics and postoperative weight loss of patients included and not included in the study were similar (data not shown). Second, all food information was based on self-reported data, and an underreporting component should be considered. Third, patients underwent two different BS techniques; unfortunately, due to sample size lacks sufficient statistical power to compare both techniques. Finally, data regarding intolerance or gastrointestinal symptoms was not recorded.
In conclusion, the present study adds evidence on the benefits of a preoperative nutritional intervention in morbidly obese patients scheduled for bariatric surgery. Although previous literature associated the changes in alimentary habits after bariatric surgery to the surgical procedure itself, our results support the great influence of the preoperative nutritional intervention on the patterns of dietary intake of these patients.
Contribution statementAll authors met authorship requirements, actively participated in data acquisition, drafting or revising the paper, contributed to the discussion and gave approval of the final version. DB and AP conceived and designed the study, collected data, performed data analysis, reviewed the manuscript and contributed to discussion. IS performed data analysis and reviewed the manuscript. JPB reviewed the manuscript and contributed to discussion MV collected data and reviewed the manuscript. JMR collected data, performed data analysis and drafted the manuscript. EC collected data, performed data analysis and drafted the manuscript and tables. JAF collected data, performed data analysis and reviewed the manuscript. AG conceived and designed the study, collected data, performed data analysis, reviewed the manuscript and contributed to discussion.
Conflict of interestThe authors have no conflict of interest to declare.
We thank Helmut Schroeder PhD for valuable assistance and Miss Christine O’Hara for review of the English version of the manuscript.
CIBEROBN and CIBERESP are an initiative of the Instituto de Salud Carlos III (ISCIII).