The purpose of this prospective study was to assess the effects of selenium supplementation on TSH and interferon-γ inducible chemokines (CXCL9, CXCL10 and CXCL11) levels in patients with subclinical hypothyroidism due to Hashimoto's thyroiditis.
Patients and methodsPatients with subclinical hypothyroidism due to Hashimoto thyroiditis were prospectively enrolled in the SETI study. They received 83mcg of selenomethionine/day orally in a soft gel capsule for 4 months with water after a meal. No further treatment was given. All patients were measured thyroid hormone, TPOAb, CXCL9, CXCL10, CXCL11, iodine, and selenium levels at baseline and at study end.
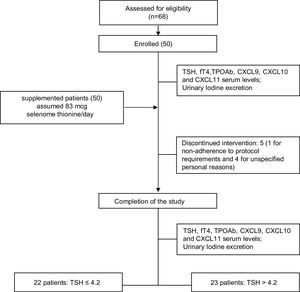
Results50 patients (43/7 female/male, median age 43.9±11.8 years) were enrolled, of which five withdrew from the study. At the end of the study, euthyroidism was restored in 22/45 (48.9%) participants (responders), while 23 patients remained hypothyroid (non-responders).
There were no significant changes in TPOAb, CXCL9, CXCL10, CXCL11, and iodine levels from baseline to the end of the study in both responders and non-responders. TSH levels were re-tested six months after selenomethionine withdrawal: 83.3% of responding patients remained euthyroid, while only 14.2% of non-responders became euthyroid.
ConclusionsThe SETI study shows that short-course supplementation with selenomethionine is associated to a normalization of serum TSH levels which is maintained 6 months after selenium withdrawal in 50% of patients with subclinical hypothyroidism due to chronic autoimmune thyroiditis. This TSH-lowering effect of selenium supplementation is unlikely to be related to changes in humoral markers of autoimmunity and/or circulating CXCL9.
El objetivo de este estudio prospectivo es evaluar los efectos de los suplementos de selenio sobre las concentraciones de TSH y de quimiocinas inducibles por interferón γ (CXCL9, CXCL10 y CXCL11) en pacientes con hipotiroidismo subclínico, debido a tiroiditis de Hashimoto.
Pacientes y métodosSe incluyó prospectivamente en el estudio SETI a pacientes con hipotiroidismo subclínico, debido a tiroiditis de Hashimoto. Recibieron 83μg de selenometionina al día por vía oral en una cápsula de gel blanda durante 4 meses con agua después de una comida. No se administró más tratamiento. Se sometió a todos los pacientes a evaluaciones del perfil hormonal tiroideo, anticuerpos anti-TPO, CXCL9, CXCL10, CXCL11, yodo y selenio en el momento del reclutamiento y al final del estudio.
ResultadosSe reclutó a 50 pacientes (43/7 mujeres/varones, mediana de edad de 43,9±11,8 años); 5 se retiraron del ensayo. Al final del estudio, 22/45 (48,9%) participantes recuperaron el eutiroidismo (respondedores) y 23 se mantuvieron hipotiroideos (no respondedores).
No se observaron diferencias significativas en los valores de anticuerpos anti-TPO, CXCL9, CXCL10 y CXCL11 y yodo entre el momento basal y el final del estudio en los pacientes con y sin respuesta. La TSH se volvió a analizar 6 meses después de la retirada de la selenometionina: el 83,3% de los sujetos con respuesta seguían siendo eutiroideos, mientras que solo el 14,2% de los que no habían respondido se convirtieron en eutiroideos.
ConclusiónEl estudio SETI muestra que la suplementación de corta duración con selenometionina se asocia con una normalización de las concentraciones séricas de TSH que se mantiene 6 meses después de la retirada del selenio en el 50% de los pacientes con hipotiroidismo subclínico debido a tiroiditis autoinmunitaria crónica. Es improbable que esta acción reductora de la TSH de los suplementos de selenio esté relacionada con cambios de los marcadores humorales de autoinmunidad o del CXCL9 circulante.
Subclinical hypothyroidism (SCH) is defined as a state of increased serum thyroid-stimulating hormone (TSH) levels, with circulating serum free thyroxine (T4) concentration within the population reference, and it is, in most cases due to Hashimoto's thyroiditis (HT). SCH is commonly classified in relation to the degree of the elevation of the serum TSH level: (i) mildly increased TSH levels (4.0–10.0mIU/L), and ii) more severely increased TSH levels (>10mIU/L). According to current clinical guidelines, treatment with levothyroxine was recommended for all SCH patients with TSH levels >10mIU/L, whereas for those with TSH levels below 10mIU/L the need for treatment remains controversial.1
Selenium (Se) is an essential trace element in all higher animal species including humans. The human selenoproteome consists in few selenoproteins. About half have so far been ascribed a biological function, but several have no specific functions.2,3 Mammalian selenoproteins can broadly be classified into housekeeping and stress-related proteins.4 The main functional proteins in the thyroid gland are antioxidative oxidoreductases (i.e. glutathione peroxidase) and thioredoxin reductases which control the redox status and protect from damage caused by oxygen free radicals and they have an essential role in the antioxidant process. In addiction two isoforms are responsible for the local activation of thyroid hormones.5
The thyroid gland presents the highest selenium (Se) concentration of all body tissues and contains many selenoproteins with specific functions. In the 1980s, it was demonstrated that Se supplementation is crucial in preventing and/or reversing the clinical signs of severe Se deficiencies.6 Ten years later, the link between severe Se deficiency and thyroid dysfunction in children was first described.7
Some studies have shown that Se supplementation may be of clinical benefit in autoimmune thyroid disorders such as, for example, in Graves’ ophthalmopathy.8–11 More recently, normalization of the serum levels of TSH in nearly a third of patients with subclinical hypothyroidism due to autoimmune thyroiditis after selenium supplementation was reported by our group.12
The potential immunomodulatory effects of selenium supplementation, in terms of modulation of the secretion of the interferon-γ inducible chemokines (CXCL9, CXCL10 and CXCL11), was tested in two previous studies with discrepant findings.13,14 Indeed, Pilli et al. in a recent randomized study, reported that the administration of selenium methionine in euthyroid women affected by autoimmunity thyroid diseases (AITD) is able to reduce the serum levels of CXCL9, CXCL10 and CXCL11.13 On the other hand, Esposito et al. reported no changes in the circulating levels of CXCL10, in untreated euthyroid patients with Hashimoto's thyroiditis receiving L-selenomethionines.14 The different clinical settings of the above studies seems a likely explanation accounting for discrepancy. The issue of the immunomodulating effect of Se, through a lowering of CXCL9, CXCL10 and CXCL11 could be of potential relevance. Indeed, these molecules are known to be involved in several autoimmune endocrine diseases including AITDs being their serum and tissue levels elevated in these patients.15,16 The above considerations lead us to further evaluate the effects of Se supplementation both in terms of circulating TSH levels and on CXCL9, CXCL10 and CXCL11 concentrations in patients with sub-clinical hypothyroidism due to HT. The final aim of the present SETI study (Selenio e Tiroide, translated as “Selenium and Thyroid”) will be to provide further insights on the immunomodulatory effects of SE in patients with AITD.
MethodsPatients aged 18–65 were eligible. Inclusion criteria were represented by the presence of: (1) mild subclinical hypothyroidism (TSH range 4.26–10.00mIU/L); (2) positive test for thyroid peroxidase autoantibody (TPOAb), as well as ultrasound pattern suggesting chronic autoimmune thyroiditis; (3) no previous or current levothyroxine treatment. In this study, pregnant women, those who were planning to become pregnant and patients in whom, according to most recent guidelines,1 levothyroxine treatment was recommended were excluded. All the subjects in the study were Caucasians, born and living in the Brescia area (Italy). All participants were otherwise healthy. Patient enrolment took place from June 2015 through February 2016. The diagnosis of HT was assessed by the presence of detectable TPOAb serum levels and by the typical ultrasound features.17,18 Thyroid sonography was performed with a real-time instrument (Vision 900; Hitachi Medical System, Tokyo, Japan) equipped with a linear probe with a central frequency of 6–13MHz, by the same skilled sonographer.
The study was designed as prospective and patients received 83mcg selenomethionine/day orally in a soft gel capsule (Syrel®, IBSA Italia) for 4 months with water after a meal. No further treatment was given. All the patients were submitted to thyroid hormonal profile (thyroid-stimulating hormone – TSH; free T4 – fT4), TPOAb, interferon-γ inducible chemokines (CXCL9, CXCL10 and CXCL11), iodine and selenium evaluations upon enrolment and at the end of the study. The blood assays were performed in the same laboratory.
The study protocol (no. 1981) was approved by the Ethics Committee of our institution (Medical School, University of Brescia). Written informed consent was obtained from all participants.
Serum fT4, TSH and TPOAb assaysSerum concentrations of fT4 (normal range: 8.0–19.0pg/mL) and TSH (third generation TSH assay; normal range: 0.27–4.20mIU/L) were measured using immuno-chemiluminescent assays by an automated analyser (Immulite 2000, DPC Cirrus, Los Angeles, CA, USA) and with commercial kits (Diagnostic Products Corporation, Los Angeles, CA, USA).
The serum concentrations of TPOAb (normal range: <60U/mL) were measured using immuno-chemiluminescent assays and with commercial kits (Brahms, Hennigsdorf, Germany).
CXCL9, CXCL10, CXCL11 serum assaysSerum samples were collected from patients and controls and stored at −20°C until the assay. Chemokines level were measured using commercially available kits (R&D Systems, Minneapolis, MN). The mean minimum detectable dose of CXCL9 was 3.84pg/mL. The intra- and inter-assay coefficients of variation were 3.3 and 8.2%, respectively. The mean minimum detectable dose of CXCL10 was 1.67pg/mL. The intra- and inter-assay coefficients of variation were 3.0 and 6.1%, respectively. The mean minimum detectable dose of CXCL11 was 13.9pg/mL. The intra- and inter-assay coefficients of variation were 4.7 and 7.6%, respectively. All samples were assayed in duplicate. Quality control pools of low, normal or high concentrations were included in each assay.
Iodine determination assayIodine was measured by an inductively coupled-plasma mass spectrometer ICP-MS (ELAN DRC II, Perkin Elmer, Waltham, USA). The urine samples were diluted 1:10 with distilled water. The addition calibration standards curve was prepared with standard solutions of Iodine ranging from 10μg/L to 1000μg/L: salt potassium iodide (KI PM 166.01; Carlo Erba Reagenti, Milan, Italy), from which was prepared the stock solution of Iodine 1mg/mL. The curve and sample solutions were pumped in the spray chamber by a peristaltic pump and the mass detected was 127. The accuracy of the method was determined on the basis of the mean values obtained on the certified reference materials Toxic Elements Urine by NIST 2670a (MD, USA). The accuracy was 96%, while the coefficient of variation (CV) was 7.0%. The limit of detection (LOD), calculated as 3 standard deviations of the background signal obtained on 10 white samples, was 1.0μg/L. The laboratory in which the study was performed participates in the external quality assessment programme and is certified for the above mentioned metallic elements by G-EQUAS of the German Society of Occupational and Environmental Medicine.
Selenium serum concentrations assaySelenium was measured by means of an Atomic Absorption Spectrometer Varian Zeeman (SpectraAA400) background-corrected with Zeeman effect.
Serum samples were diluted 1:3 with a Triton X-100 0.05%. The addition calibration standards curve was prepared with standard solutions of Selenium ranging from 50μg/L to 100μg/L: selenium in HNO3 2% mono elemental standard (Chemical Research 2000, SC, USA). The modifier of the matrix was Palladium 1000ppm in HNO3 2% mono elemental standard (Chemical Research 2000, SC, USA).
The accuracy of the method was determined on the base of the mean values obtained on certified reference materials metallic elements by G-EQUAS of the German Society of Occupational and Environmental Medicine. The Se accuracy was 95%, while the coefficient of variation (CV) was 5.1%
The limit of detection (LOD), calculated as 3 standard deviations of the background signal obtained on 10 white samples, was 10μg/L.
Statistical analysisStatistical analysis was performed using the SPSS software 17.0 (SPSS, Inc., Evanston, IL). Differences among patients (namely Responders and non-responders), at baseline or at the end of the study, were evaluated by analysis of variance (ANOVA). To evaluate the influence of biochemical parameters obtained at the study entry on prognosis, i.e. euthyroidism vs hypothyroidism, logistic regression analysis was used. The sensitivity and the specificity of basal TSH cut-off were determined by the Receiver Operating Characteristic (ROC)-curve method.
Values are presented as mean±standard deviation (SD). A p-value <0.05 was considered statistically significant.
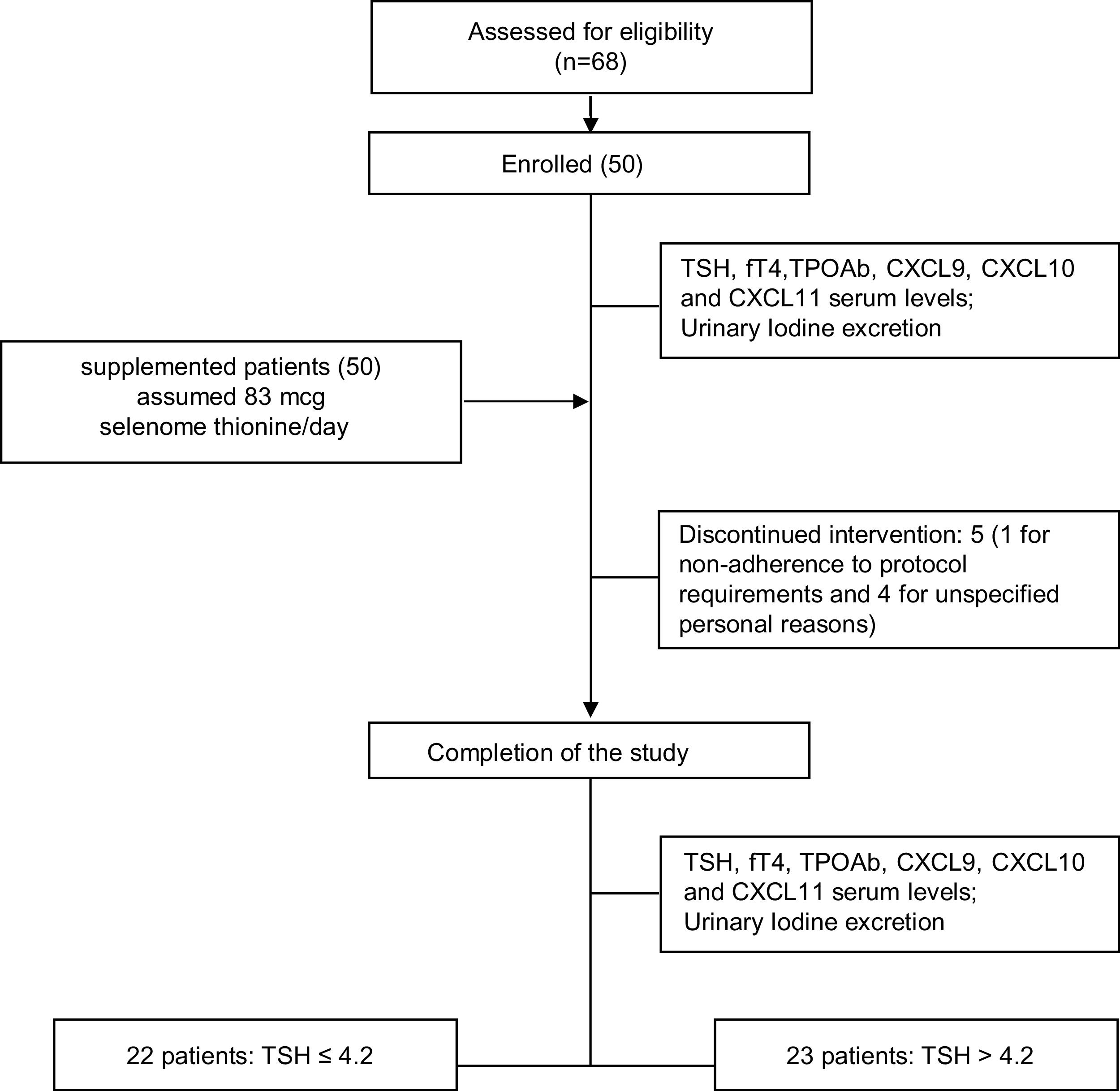
ResultsPatients: baseline demographics and clinical characteristicsSixty-eight patients were assessed for eligibility and 50 patients (43/7 female/male, median age 43.9±11.8 years) were recruited and enrolled in the study. Five out of 50 patients withdrew from the trial: in one case due to non-adherence to protocol requirements and in the remaining four cases for unspecified personal reasons (Table 1). The study flowchart is shown in Fig. 1
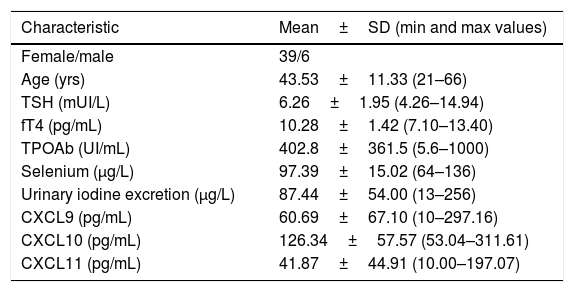
Baseline demographics and clinical characteristics of the patients in the study.
| Characteristic | Mean±SD (min and max values) |
|---|---|
| Female/male | 39/6 |
| Age (yrs) | 43.53±11.33 (21–66) |
| TSH (mUI/L) | 6.26±1.95 (4.26–14.94) |
| fT4 (pg/mL) | 10.28±1.42 (7.10–13.40) |
| TPOAb (UI/mL) | 402.8±361.5 (5.6–1000) |
| Selenium (μg/L) | 97.39±15.02 (64–136) |
| Urinary iodine excretion (μg/L) | 87.44±54.00 (13–256) |
| CXCL9 (pg/mL) | 60.69±67.10 (10–297.16) |
| CXCL10 (pg/mL) | 126.34±57.57 (53.04–311.61) |
| CXCL11 (pg/mL) | 41.87±44.91 (10.00–197.07) |
At the end of the study 22/45 (48.9%) participants restored euthyroidism (responders) and 23 remained hypothyroid (non-responders). The biochemical findings compared between and within responders and non-responders at baseline and at the end of the study are reported in Tables 2 and 3.
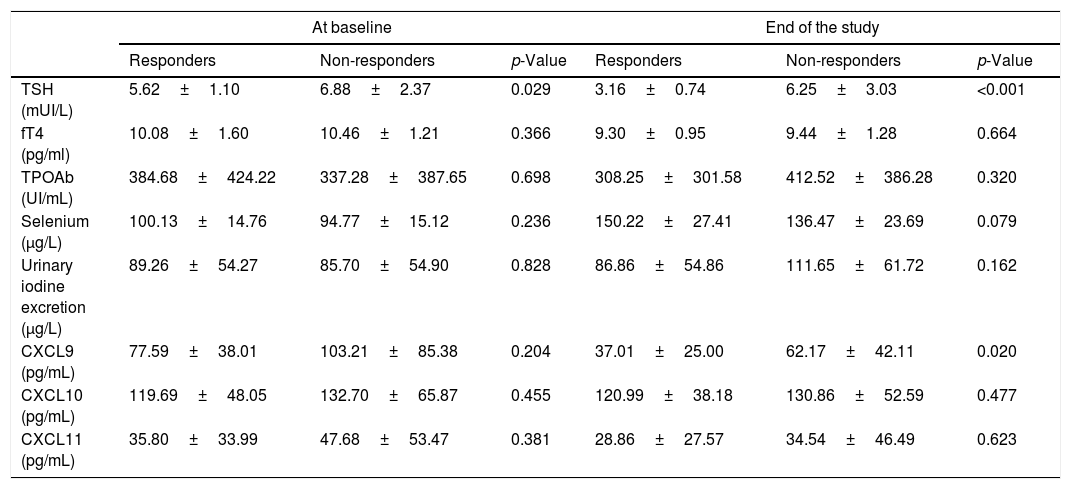
Biochemical characteristic variation of responders vs non-responders at baseline and at the end of the study. Values are expressed as mean±SD.
| At baseline | End of the study | |||||
|---|---|---|---|---|---|---|
| Responders | Non-responders | p-Value | Responders | Non-responders | p-Value | |
| TSH (mUI/L) | 5.62±1.10 | 6.88±2.37 | 0.029 | 3.16±0.74 | 6.25±3.03 | <0.001 |
| fT4 (pg/ml) | 10.08±1.60 | 10.46±1.21 | 0.366 | 9.30±0.95 | 9.44±1.28 | 0.664 |
| TPOAb (UI/mL) | 384.68±424.22 | 337.28±387.65 | 0.698 | 308.25±301.58 | 412.52±386.28 | 0.320 |
| Selenium (μg/L) | 100.13±14.76 | 94.77±15.12 | 0.236 | 150.22±27.41 | 136.47±23.69 | 0.079 |
| Urinary iodine excretion (μg/L) | 89.26±54.27 | 85.70±54.90 | 0.828 | 86.86±54.86 | 111.65±61.72 | 0.162 |
| CXCL9 (pg/mL) | 77.59±38.01 | 103.21±85.38 | 0.204 | 37.01±25.00 | 62.17±42.11 | 0.020 |
| CXCL10 (pg/mL) | 119.69±48.05 | 132.70±65.87 | 0.455 | 120.99±38.18 | 130.86±52.59 | 0.477 |
| CXCL11 (pg/mL) | 35.80±33.99 | 47.68±53.47 | 0.381 | 28.86±27.57 | 34.54±46.49 | 0.623 |
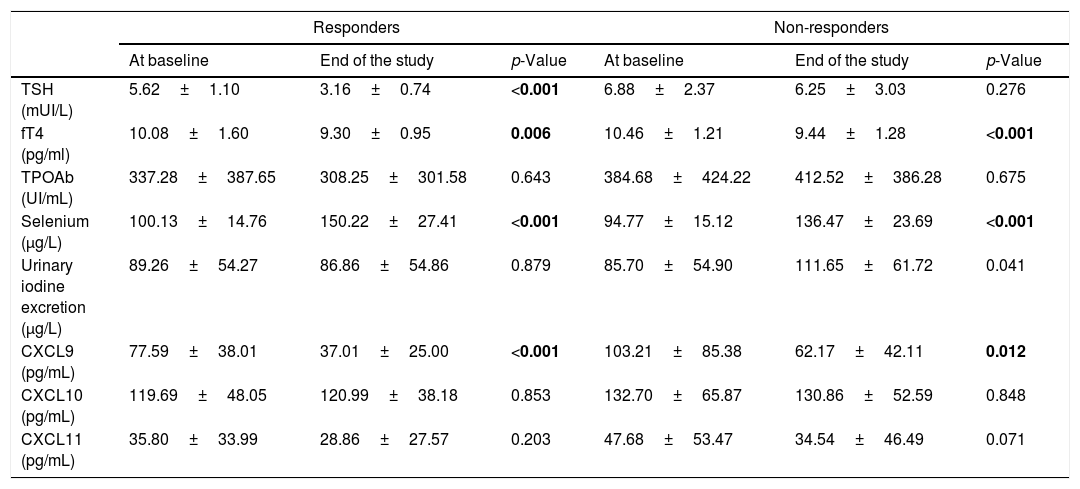
Biochemical characteristic variations among from baseline and to the end of the study in responders and non-responders. Values are expressed as mean±SD. Statistically significant values are indicated in bold.
| Responders | Non-responders | |||||
|---|---|---|---|---|---|---|
| At baseline | End of the study | p-Value | At baseline | End of the study | p-Value | |
| TSH (mUI/L) | 5.62±1.10 | 3.16±0.74 | <0.001 | 6.88±2.37 | 6.25±3.03 | 0.276 |
| fT4 (pg/ml) | 10.08±1.60 | 9.30±0.95 | 0.006 | 10.46±1.21 | 9.44±1.28 | <0.001 |
| TPOAb (UI/mL) | 337.28±387.65 | 308.25±301.58 | 0.643 | 384.68±424.22 | 412.52±386.28 | 0.675 |
| Selenium (μg/L) | 100.13±14.76 | 150.22±27.41 | <0.001 | 94.77±15.12 | 136.47±23.69 | <0.001 |
| Urinary iodine excretion (μg/L) | 89.26±54.27 | 86.86±54.86 | 0.879 | 85.70±54.90 | 111.65±61.72 | 0.041 |
| CXCL9 (pg/mL) | 77.59±38.01 | 37.01±25.00 | <0.001 | 103.21±85.38 | 62.17±42.11 | 0.012 |
| CXCL10 (pg/mL) | 119.69±48.05 | 120.99±38.18 | 0.853 | 132.70±65.87 | 130.86±52.59 | 0.848 |
| CXCL11 (pg/mL) | 35.80±33.99 | 28.86±27.57 | 0.203 | 47.68±53.47 | 34.54±46.49 | 0.071 |
At baseline, the two groups were superimposable for all the considered parameters with the exception of the TSH value, which was lower in responders than in non-responders (5.62±1.10 vs 6.88±2.37mIU/L, respectively, p=0.029) (Table 2). However, at the end of the study, the percentage of the TSH decrease was significantly greater among responders compared to non-responders (42.6% vs 6.8%, respectively; p<0.001) (Table 2). Serum Se concentration increased similarly in the two groups from baseline to the end of the study (54% vs 45%, Responders vs Non Responders.
A multivariate logistic regression model was constructed by entering the dichotomous variable (Responder/Non-Responder) as a dependent variable, while age, TSH, fT4, TPOAb, Selenium (μg/L), UIE (μg/L), CXCL9, CXCL10 and CXCL11 all served as covariates.
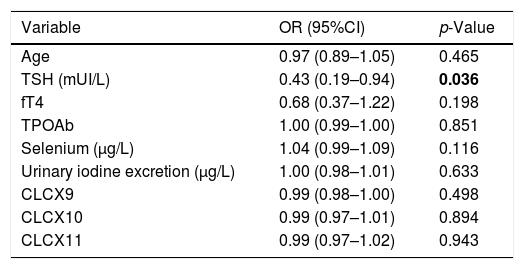
The results of the logistic regression analysis showed that, among the parameters evaluated at the study entry, TSH serum levels was the only statistically significant predictor for reaching euthyroidism at the end of the study (Table 4). Its prognostic value was evaluated by the ROC analysis which indicated 5.38mUI/L as the best TSH cut-off value. In fact, initial TSH levels ≤5.38mUI/L identified patients who had achieved euthyroidism with a sensitivity of 50% and specificity of 69.6%. No differences in the TPOAb titres were observed from baseline to the end of the study, whereas serum fT4 significantly decreased both in responders (10.08±1.60 vs 9.30±0.95, p=0.006) and non-responders (10.46±1.21 vs 9.44±1.28, p<0.001) (Table 3). Finally, no significant differences were observed among the two groups in the UIE levels both at baseline and after administration of Se.
Multivariate logistic regression model values. Statistically significant values are indicated in bold.
| Variable | OR (95%CI) | p-Value |
|---|---|---|
| Age | 0.97 (0.89–1.05) | 0.465 |
| TSH (mUI/L) | 0.43 (0.19–0.94) | 0.036 |
| fT4 | 0.68 (0.37–1.22) | 0.198 |
| TPOAb | 1.00 (0.99–1.00) | 0.851 |
| Selenium (μg/L) | 1.04 (0.99–1.09) | 0.116 |
| Urinary iodine excretion (μg/L) | 1.00 (0.98–1.01) | 0.633 |
| CLCX9 | 0.99 (0.98–1.00) | 0.498 |
| CLCX10 | 0.99 (0.97–1.01) | 0.894 |
| CLCX11 | 0.99 (0.97–1.02) | 0.943 |
Interestingly, serum levels of the chemokine CXCL9 changed after Se administration in comparison to baseline. In particular, CXCL9 serum levels significantly decreased in responders (77.59±38.01 baseline vs 37.01±25.00 after selenium supplementation, p<0.001) and in non-responders (baseline 103.21±85.38 vs 62.17±42.11 after selenium supplementation, p=0.012) (Table 3). Moreover, significant differences were observed also between responders and non-responders after Se supplementation (37.01±25.00 responders vs 62.17±42.11 non-responders; p=0.020).
On the contrary, no significant changes were observed in CXCL10 and CXCL11 levels from baseline to the end of the study and between responders and non-responders (Table 3).
Long-term TSH follow-upThe serum levels of TSH were re-tested in all patients after six months after selenomethionine withdrawal. Among responders four patients were lost at follow-up whereas two women in the non-responders group were started on levothyroxine substitution treatment because of pregnancy planning. Serum TSH (mUI/L) resulted significant lower among responder than non-responders group (3.63±0.86 vs 7.41±1.24, respectively, p<0.0001). In details, 15/18 (83.3%) patients remained in euthyroidism among responder while only 3/21 (14.2%) became euthyroid in the Non responder group.
DiscussionThe present study confirms and extends a previous one12 showing that a short term of Se supplementation is able to restore euthyroidism in a large number of patients affected by subclinical hypothyroidism due to chronic autoimmune thyroiditis. In the last few years, some studies aimed at evaluating the effects of selenium supplementation in different thyroid disorders were performed, but rather discrepant results were provided.8–13,19–22
One of the reason of this debate is the fact that spontaneous recovery has been reported in patients affected by subclinical hypothyroidism due to chronic autoimmune thyroiditis, although the frequency of this phenomenon remains unclear23,24 and it seems to be more frequent in subjects without thyroid peroxidase antibodies and within the first two years since diagnosis.25 On the contrary, in a consistent percentage of patients, untreated SCH may progress to overt hypothyroidism with a cumulative incidence ranging from 33% to 55% in 10 to 20 years of follow-up.23
Indeed, a recent meta-analysis by Wichman et al. showed that selenium supplementation reduced serum TPOAb levels both in LT4-treated that untreated patients with chronic autoimmune thyroiditis even if serum TSH improvement remains to be demonstrated.26 A possible explanation of these discrepant results could be also due to the different regimens of selenium supplementation adopted in different studies as well evidenced by Turker et al.27 and Kvicala and colleagues.28
However, to the best of our knowledge, only two previous studies investigated the influence of Se supplementation in sub-clinically hypothyroid patients affected by Hashimoto's thyroiditis, a condition in which genetic predisposition, immunological and environmental factors including selenium deficiency are potentially involved.12,21
Nordio and colleagues have showed a more frequent restoration of TSH levels among patients treated with both Se and myo-inositol supplementation than those who received only selenium.21 Also, as previously mentioned, our group showed that Se supplementation can restore euthyroidism in one third of subclinical hypothyroid patients affected by Hashimoto's thyroiditis.12 The present study confirms and extends the previous data showing a restoration of euthyroidism in 50% of patients after 4 months of Se supplementation. More importantly, the results of the present study, demonstrate that in more than 80% of the patients receiving selenium who had normalized their serum level of TSH the effect of selenium was maintained after six months from selenomethionine withdrawal. These last data, in particular the long follow up of patients who had normalized their serum level of TSH, strengthen our hypothesis that selenium supplementation is able to restore euthyroidism and that the TSH normalization is not due to a spontaneous recovery.
It is important to highlight that the benefits of selenium supplementation occurred preferentially in those patients displaying lower degrees of TSH elevation at baseline and that the percentage of reduction of the basal serum TSH levels was significantly greater in responders as compared to non-responders (42.6% vs 6.8%; p<0.001). In addition, the restoration of euthyroidism was not related to changes in the serum TPO Ab titres, which did not change significantly in both groups throughout the study. This aspect deserves being discussed as it remains controversial. The issue of whether Se administration may lead to changes in the circulating levels of IFNγ- inducible chemokines was previously addressed by two studies reporting contrasting findings. Briefly, Pilli and colleagues found a significant and stable reduction of CXCL9 and CXCL10 in course of selenomethionine supplementation in women with Euthyroid Autoimmune Thyroiditis (EAT), suggesting an immunomodulatory effect of selenium administration.13 On the other hand, Esposito and colleagues, found no changes in CXCL10 levels after administration of l-seleniometionine in untreated EAT patients.14 The results of the current study show that among the three chemokines CXCL9 CXCL10 CXCL11, the effect of Se administration was evident only for CXCL9, which levels decreased in sub-clinically hypothyroid patients with HT, but not for CXCL10 and CXCL11. However, changes in CXCL9 levels were observed in both Responder and Non-responder patients. Indeed, the results of the logistic regression analysis showed that the only statistically significant parameter for normalization of serum TSH levels in course of Se supplementations was the TSH serum at baseline, being 5.38mIU/L the best TSH cut-off value as assessed by ROC curves.
A further issue to discuss is the timing of evaluation of the effect of Se on the circulating levels of chemokines, which has been reported by others to be significantly reduced for serum CXCL9, CXCL10 and CXCL11 after 12 months of Se supplementation,8 while, in this study, after 4 months of Se administration, this lowering effect was limited to CXCL9.
In conclusion, the results of the present study show that a short-course supplementation with selenomethionine is associated with a normalization of serum TSH levels which is maintained 6 months after selenium withdrawal, in 50% of the patients with subclinical hypothyroidism due to chronic autoimmune thyroiditis. This data could be of some interest by the fact that the need for thyroxine treatment of patients with subclinical hypothyroidism remains controversial.1 In this case, supplementation with selenium could be a good compromise between giving or not giving therapy with levothyroxine. Further prospective large trials are needed to clarify this important argument.
Conflict of interestThe authors have nothing to declare.