Type 1 diabetes mellitus (DM1) is characterised by autoimmune destruction of the pancreatic beta cell, and pancreatic autoimmunity markers are therefore useful in the diagnosis of the disease.1 Since it was identified in 2007,2 the anti-zinc transporter 8 (anti-ZnT8) antibody has become one of the markers of autoimmunity against pancreatic islet cells, along with anti-glutamate acid decarboxylase (anti-GAD) and anti-tyrosine phosphatase antibodies AI-2 (anti-IA-2).3 However, it is not used on a generalised basis in Spanish hospitals. In April 2017 anti-ZnT8 was included in the region of Asturias as part of the autoimmune study into DM1. The aim of this study was to determine pancreatic autoimmunity status in diagnoses of DM1, with special attention to the utility of anti-ZnT8. We also looked at whether or not to perform the assessment of pancreatic autoimmunity in a stepwise manner.
Data from patients diagnosed with DM1 in Asturias were collected from when anti-ZnT8 was introduced into the assessment protocol until December 2020. The antibodies were considered positive with the following titres: anti-GAD >10, anti-IA-2 > 10 and/or anti-ZnT8 > 20 units/ml, as measured by RSR ELISA (Cardiff, United Kingdom). We identified how many of these new diagnoses had anti-ZnT8, anti-GAD or anti-IA-2 as the only element of autoimmunity. We compared the clinical characteristics of the patients with anti-ZnT8 to the negative patients. Lastly, we established the proposal for automated stepwise assessment with an initial assessment of anti-GAD, a subsequent study of anti-IA-2 in the case of negative anti-GAD, and a final analysis of anti-ZnT8 if the two previous tests are negative. The study was approved by the Principality of Asturias Independent Ethics Committee (project number 2020.323).
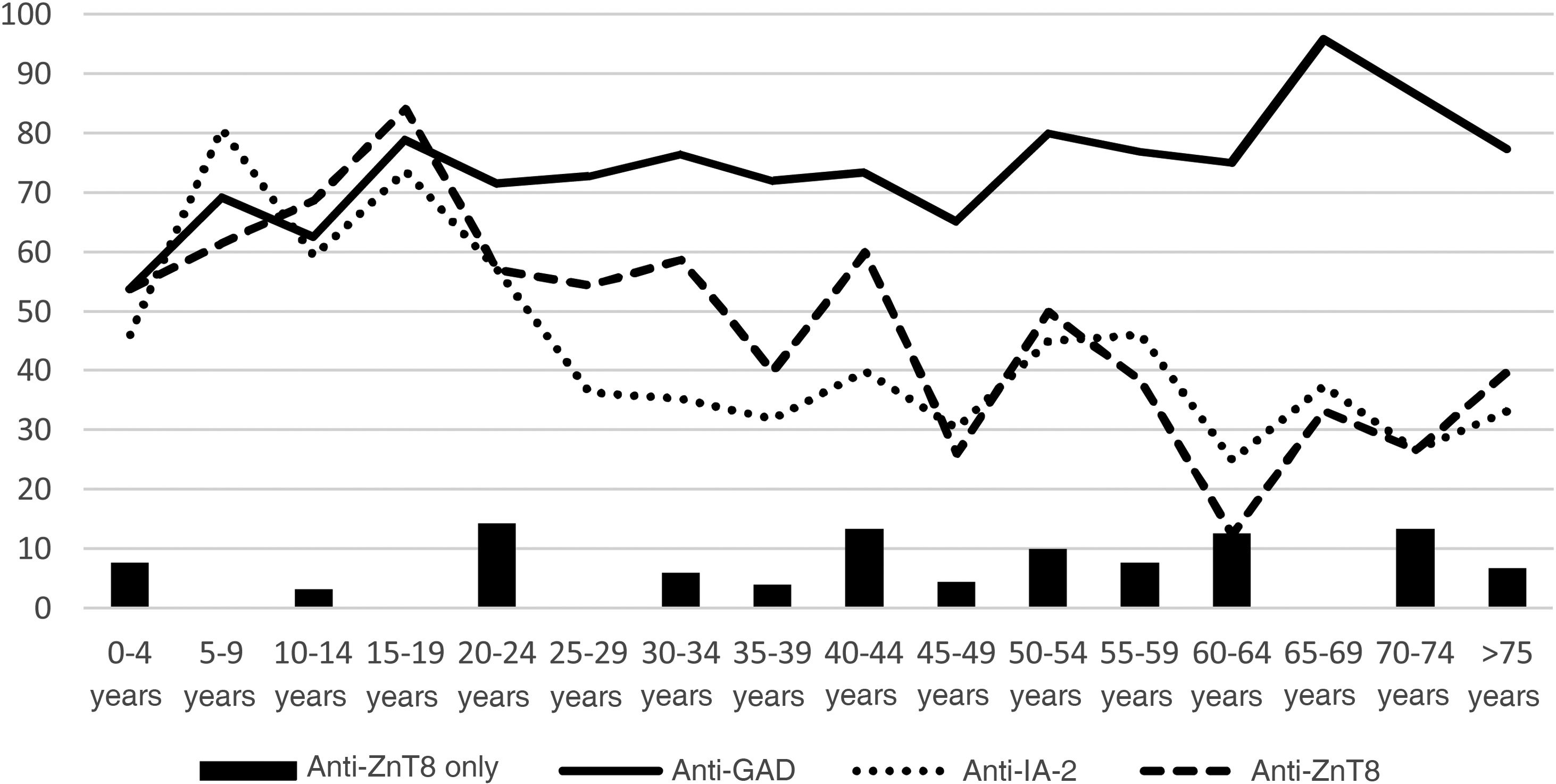
Of the 304 patients diagnosed with DM1, 94.41% had pancreatic autoimmunity. The prevalence of anti-GAD was 74.01%, anti-IA-2, 45.07% and anti-ZnT8, 48.03%. Fig. 1 shows the distribution according to age groups.
In 5.59% of the patients, anti-ZnT8 was the only positive antibody, while 31.58% were only positive for anti-GAD and 6.91% only for anti-IA-2. By age groups, represented in the figure, 2.22% of patients under 20, 5% of those aged 20–40, and 7.79% of those over 40 had anti-ZnT8 autoimmunity only.
In patients with anti-ZnT8 autoimmunity, the age at diagnosis was lower than in those without anti-ZnT8 (32.76 ± 22.76 vs 45.49 ± 22.57, p < 0.0001). No statistically significant differences were found in HbA1c at baseline (11.08% vs 11.17%, p: 0.811); diabetic ketoacidosis (22.55% vs 30%, p: 0.679); BMI (22.17 vs 21.87 kg/m2, p: 0.511); C-peptide (0.99 vs 1.15 ng/ml, p: 0.493); personal history of autoimmune thyroid disease or coeliac disease (12.75% vs 11.27%, p: 0.818); or in a family history of DM1 (23.16% vs 27.69%, p: 0.579).
With the proposal for the stepwise testing for pancreatic autoimmunity, 225 of the 304 patients diagnosed with DM1 had anti-GAD autoimmunity, so no further studies would be required. Of the remaining 79 patients, 45 were positive for IA-2. Therefore, performing the analyses in stages, 74.01% of the anti-IA-2 tests and 88.82% of the anti-ZnT8 tests would have been avoided.
Our study shows that pancreatic autoimmunity is confirmed in most people diagnosed with DM1. Anti-GAD is the most prevalent antibody, being much more common than anti-IA-2 and anti-ZnT8 in the over 20 s. Other studies point to anti-GAD as the main marker of pancreatic autoimmunity in adults4; for this reason, the American Diabetes Association and European Association for the Study of Diabetes consensus for the diagnosis of DM1 in adults recommends that it be studied first.5
The prevalence of anti-ZnT8 in our sample behaves similarly to that of anti-IA-2, with a predominance in children and decreasing in adulthood, in line with the findings of other studies.6,7 Moreover, faster conversion to negative has been reported with anti-ZnT8 than with anti-GAD and anti-IA-22.2 It is worth noting that only 5.59% of the patients had anti-ZnT8 as the only antibody, suggesting that the vast majority of patients could have been diagnosed with DM1 without the need for this test.
The prevalence of anti-ZnT8 is higher in younger age groups, although we found no other differences in the clinical characteristics of patients with anti-ZnT8 autoimmunity compared to those without anti-ZnT8. This is in line with other studies, which also indicate that there are no changes in the prognosis of the disease.7–9
Anti-ZnT8 is therefore an antibody which in most cases does not provide additional information in the diagnosis of DM1, is not related to differential clinical characteristics in patients and, according to other studies, does not change the prognosis. These findings invite both reflection and further study into the utility of measuring anti-ZnT8. As the presence of a single marker of pancreatic autoimmunity in a person with diabetes mellitus indicates autoimmune aetiology, and with it the diagnosis of DM1,5,10 the option of performing the study of autoimmunity in a stepwise manner should be considered. Thus, anti-ZnT8 would only be analysed if the patient is negative for anti-GAD and anti-IA-2. In our sample, 88.82% of the tests could have been avoided. Furthermore, given the prevalence of anti-GAD, much of the anti-IA-2 assays could also have been avoided. This means the technique can be applied more efficiently and reduces healthcare costs.
In conclusion, the presence of pancreatic antibodies is confirmed in most new diagnoses of DM1. Anti-GAD has the highest prevalence, especially in adults. Anti-ZnT8 is useful in a limited number of patients and is not associated with differential clinical characteristics, so ways should be sought to increase the effectiveness of measuring this antibody. Stepwise assessment of pancreatic autoimmunity may be a useful strategy to reduce unnecessary testing and thereby help control healthcare spending.