Exposure to seasonal environmental factors during gestation or early in the postnatal period could influence the development of autoimmunity, determining a seasonality in the month of birth (MOB). There are studies evaluating this potential seasonality in patients with type 1 diabetes (T1D), autoimmune thyroid diseases (AITD), and Addison's disease (ADD), but results have been controversial.
MethodsSystematic review according to PRISMA guidelines, using PubMed, Web of Science and WorldCat databases (2005–2020) of studies that explored the association between the seasonality of the MOB and T1D, AITD and ADD. Information on sex and age, location, methodology and internal quality, seasonal patterns, hypotheses and other factors proposed to explain seasonality were extracted. Differences in season and month of birth were further discussed.
ResultsThe initial search retrieved 300 articles, and after further screening, 11 articles fulfilled inclusion criteria and were finally selected and reviewed. 73% found a seasonal pattern and 64% showed birth peaks in spring and/or summer. Hashimoto's thyroiditis and women exhibited a higher seasonality. Ultraviolet radiation, Vitamin D levels and viral infections were identified as influencing factors.
ConclusionsThe effect of certain seasonal factors during foetal development, reflected by the seasonal differences in the MOB, could contribute to the development of endocrine autoimmune diseases in predisposed patients. Further research is needed to elucidate the mechanisms underlying the observed seasonality.
La exposición a factores ambientales durante la gestación o el periodo neonatal precoz puede condicionar el desarrollo de autoinmunidad, determinando una estacionalidad en el mes de nacimiento (MDN). Existen estudios que evalúan la potencial estacionalidad en pacientes con diabetes tipo 1 (DM1), enfermedad tiroidea autoinmune (ETAI) y enfermedad de Addison (ADD), con resultados controvertidos.
Material y métodosRevisión sistemática, siguiendo las directrices PRISMA en los buscadores PubMed, Web of Science y WorldCat (2005-2020), de los estudios que exploran la asociación entre la estacionalidad del MDN y el desarrollo de DM1, ETAI y ADD. Se recogió información relativa al sexo, edad, localización geográfica, metodología, calidad, patrón estacional, hipótesis y otros factores potencialmente asociados.
ResultadosTras una primera selección de 300 artículos, se incluyeron finalmente 11 que cumplían los criterios de inclusión. En el 73% se encontró un patrón estacional y en el 64% se encontró un pico de nacimientos en primavera y/o verano. La estacionalidad fue más evidente para la tiroiditis de Hashimoto y las mujeres. La radiación ultravioleta, vitamina D y las infecciones virales se identificaron como factores influyentes.
ConclusiónEl efecto de algunos factores estacionales durante el desarrollo fetal, reflejo de las diferencias estacionales en el MDN, puede contribuir al desarrollo de enfermedades endocrinas autoinmunes en individuos predispuestos. Se necesitan más estudios para dilucidar los mecanismos subyacentes que expliquen dicha estacionalidad.
The endocrine system may be affected by several autoimmune diseases with variable impact and severity. Autoimmune thyroid disorders (AITD) (for instance, Hashimoto's thyroiditis (HT) and Graves’ disease (GD)) and type 1 diabetes mellitus (T1D) are the most common autoimmune endocrine disorders, while adrenalitis represents the vast majority of cases of primary hypocortisolism or Addison's disease (ADD). Autoimmune endocrine diseases (AED) can coexist in the same individuals and cluster in families.1 This suggests a potential common underlying pathogenesis, mainly characterised by an aberrant immune response, which leads to loss of tolerance to organ-specific self-antigens.2
The initial trigger for autoimmune diseases has not been unequivocally identified, but several genetic factors, for instance, susceptibility genes like HLA, CTLA-4 and PTPN22, have been identified as closely implicated.3,4 In parallel, an environmental influence is suggested by an incomplete concordance in the occurrence of these diseases in monozygotic twins.5,6 In fact, several features, such as low birth weight, iodine intake, selenium deficiency, radiation, tobacco, environmental toxics, drugs, stress, microbiota and bacterial or viral infections, have all been linked to the development of autoimmune diseases.7–9
In addition, some studies have identified a trend for a fluctuant seasonality in the occurrence of autoimmune diseases, presumably due to seasonality of the related environmental factors,10 even if they occur before birth.11 In line with these hypotheses, a potential seasonality in the month of birth (MOB) in individuals with autoimmune diseases, in comparison to the general population, has been suggested,12 awakening the need for further investigation on the influence of environmental issues during the perinatal period.
In the specific setting of AED, studies on the seasonality of the MOB in patients with AITD, T1D and ADD have been performed separately and with inconsistent results. Thus, the aim of this study is to systematically review the evidence on the seasonality patterns on the MOB in patients with AED and analyse the suggested factors and hypothesis to explain such seasonality.
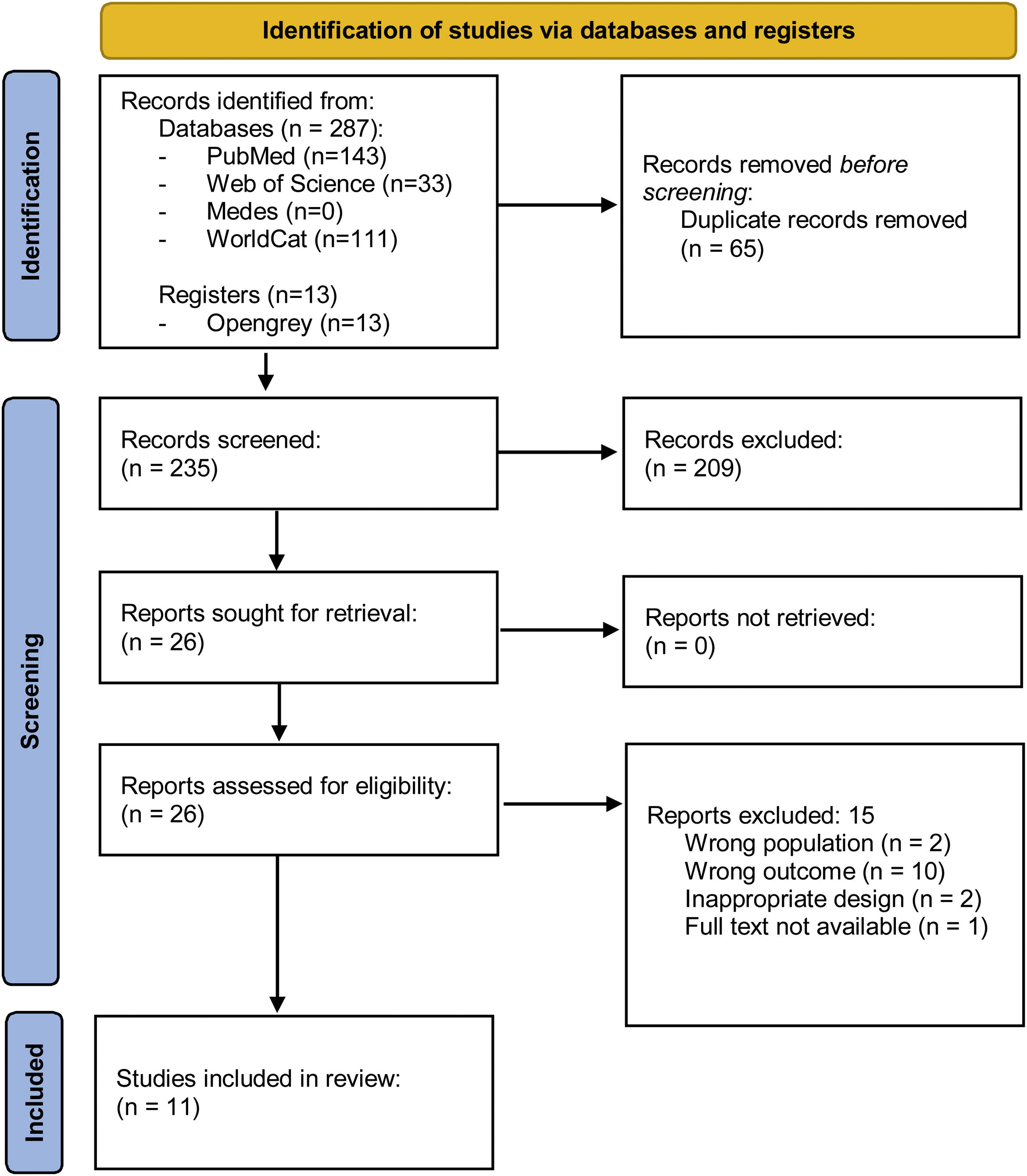
MethodsLiterature search and screeningThis systematic review followed the updated PRISMA guidelines (http://www.prisma-statement.org/).13 We used PubMed, Web of Science, Medes and WorldCat. The final search was conducted on 20 April 2020, and was restricted to full-text articles published between 2005 and 2020. Key search terms were “seasonality”, “season”, “month of birth”, “autoimmune diseases”, “endocrinology”, “autoimmune thyroid disease”, “Addison's disease”, “diabetes autoimmune”, “diabetes type 1”, and combinations thereof. We gathered 300 studies in total. We also searched for grey literature on web platforms, and found 10 book chapters and 13 theses. We also investigated the existence of additional studies by searching the citations referenced in the articles listed in PubMed which were more closely related to our topic, by using the combination of key words “seasonality” and “month of birth”. Of the 11 articles revised, we did not find any new relevant studies that had not already been included in previous search steps.
Study selection and data managementStudy selection criteria following the acronym PICOS were:
- -
Population. Patients diagnosed with AED, including HT, GD, T1D and ADD.
- -
Intervention. Observational prospective or retrospective studies which explored the seasonality in the MOB in patients with the above-mentioned diseases.
- -
Control. Healthy individuals without AED. The use of control population was not required.
- -
Outcome. Seasonality in the MOB of patients with AED.
- -
Study design. Observational prospective or retrospective study.
Exclusion criteria were:
- -
Background article. Articles included in the introduction to set the context of the study, but not related to the outcome of interest in this review; for instance, articles that comment in general on the epidemiology and aetiology of the diseases that will be examined, or references to studies performed in non-endocrine diseases.
- -
Foreign language. The search was limited to articles published in English and Spanish.
- -
Wrong outcome. Studies that did not explore the seasonality in the MOB.
- -
Wrong population. Studies not performed in humans, or studies that did not evaluate the diseases relevant to our search, i.e., non-autoimmune diseases, non-endocrine diseases, endocrine diseases different from autoimmune thyroid disease, Addison's disease or type 1 diabetes.
- -
Full-text not available, or lack of information necessary for the purpose of our study.
The Mendeley application software for data management was used to identify and exclude duplicate studies, yielding 235 studies. Screening of articles was carried out by two reviewers (GC and ARL). First, title and abstract were screened by a single reviewer (GC), and obtained 209 studies. Full-text screening was then performed by the two reviewers (CG and ARL), who individually and systematically verified that articles fulfilled all inclusion criteria and no exclusion criteria. Disagreements were resolved by consensus. A total of 11 articles were finally selected for the review. A detailed description of the search strategy used and the selection process is depicted in Fig. 1.
Data extraction and analysisRelevant information gathered from all retrieved studies was collected in a data sheet form and summarised in Table 1. Data included study design, sample size, patients’ sex, age, geographic location and latitude, AED, MOB, and date of disease diagnosis, amongst other relevant results. Seasonality of the MOB was identified as a primary or secondary aim in each study. Outcome measures were collected as follows: relative risk (RR) or odds ratio (OR) of developing the specific disease for each MOB, with their corresponding 95% confidence intervals (CI); Walter-Elwood test to determine the difference in the distribution of the MOB during the year; cosine function to evaluate the rhythmicity in the MOB; hazard ratio (HR) to establish the individual risk of each disease associated to each MOB using Cox regression models with 95% CI; and Lancaster's p-mid, as a variant of Fisher's exact test. Results for the systematic review are described individually.
Summary of the main characteristics of the studies included in this systematic review (author's alphabetical order).
| Study (author, year) | Geographic location | Patients’ database | Design | Disease | Number of patients and age | Seasonality outcome | Method and outcome measure | Seasonality and peak | Additional outcomes evaluated | Quality rating |
|---|---|---|---|---|---|---|---|---|---|---|
| Elliot et al., 2010 | Australia | Australian National Diabetes Register | Case register | T1D | 47730–14 y | Secondary | Poisson regression modelRR of developing T1D in each month (CI 95%) | No | Lower incidence with UV exposure<4000J/m2 (RR 0.85).No association with sex.Association with diagnosis in winter (RR 1.22, p<0.001) | 67 |
| Hamilton et al., 2014 | UK, Denmark, Holland | National UK Caucasian AITD Case Control Collection,National UK Caucasian GD Family Collection, OXAGEN AITD Caucasian Family Collection | Case-control | GD and HT | 3870 GD, 1219 HT and 1,424,737 controlsAdults | Primary | Walter-Elwood Test(significant if>5.991, p<0.05) | Only for the OXAGEN AITD Caucasian Family Collection:Autumn (Oct) and winter (Jan) (Walter test=7.47, p=0.02) | 78 | |
| Krassas et al., 2007 | Greece (Thessaloniki) | Endocrine Outpatient Department of Panagia General Hospital, Thessaloniki | Case-control | GD and HT | 1023 (359 GD and 664 HT)Adults | Primary | Cosinor analysis Cosine functions,Correlation tests | GD ♂: March (R 0.43)GD ♀: April and Sept (R 0.67)HT ♂: July (R 0.73)HT ♀: Jan and July (R 0.74) | In ♀ with HT seasonality in the MOB was only observed for those with high TPOAb (>1000U/mL) | 100 |
| Kyrgios et al., 2018 | Greece | Papageorgiou General Hospital | Case-control | HT | 2980–21 years | Primary | X2OR (CI 95%) of developing HT according to MOB and season | HT for MOB: March (OR 2.34, p=0.005) and Spring (OR 0.01, p=0.001)HT ♂: March (OR 3.26, p=0.015), Spring (OR 2.06, p=0.021)HT ♀: Spring (OR 1.93, p=0.01) (no significance for MOB) | 100 | |
| Laron et al., 2005 | - Europe (Italy, Ireland, Belgium, Germany, Slovenia)- USA (Pittsburg, Denver, St Louis).- Australia, New Zealand.- Israel | Previous dataa | Case-control | T1D0–14 y | 12,115:Europa (n=4842), USA (n=4509) Australia (n=621), NZ (n=275) Israel (n=1868) | Primary | Cosinor analysis Cosine functions,Correlation tests | Europe:- Sardenya: Summer- Sicily: Summer- Ireland: Summer (♂)- Slovenia: Summer- Berlin: ↓ autumn- Baden- Wuertttenberg: ↓ Spring and summerUSA:- St. Louis: Caucasian in summer (June); Afro-Americans in autumn (Sept)- New Zealand in spring (Oct)- Israel: Ashkenazi Jews in spring (May) and autumn (Oct); Arab Jews in winter (Feb) and autumn (Sept)- No seasonality for Australia, Denver and Pittsburgh. | Seasonality in the MOB only for homogeneous populations (Jewish Israel Europe, New Zealand) | 56 |
| Lewy et al., 2008 | SwedenGermany (Berlin) | Diabetes Incidence Study in Sweden. Not specified for the Berlin cohort. | Case-control | T1DSweden:15–34 y; Berlin: 1–18 y | 1077 (Sweden 572 and Berlin 505) | Primary | Cosinor analysis Cosine functions,Correlation tests | Sweden: ♂ in summer (June); ♀ in Spring (May) and summer (June).Berlin: ♂ in winter (February), spring (May) and autumn (Sept); ♀ in Spring (March) and autumn (Sept). | Seasonality in the MOB only for populations with high titers of specific antibodies | 78 |
| Mikulecky et al., 2016 | Slovakia (Bratislava) | 3rd. Department of Internal Medicine, Faculty of Medicine, Comenius University in Bratislava | Case-control | T1D and T2D | 81 patients with T1D (67 adults and 14 aged 15–18 y) | Primary | Cosinor analysis | Teenagers: Spring and early autumn;Adults: late autumn and winter | Differences in seasonality for T1D and T2D (for T2D only annual rhythm for ♀).Peak incidence of Coxsackie infection in summer and autumn | 56 |
| Pazderska et al., 2016 | UKPoland | Institute of Genetic Medicine, Newcastle University, UK. Department of Endocrinology, Metabolism and Internal Medicine, Poznan University of Medical Sciences, Poland | Case-control | AAD | 646 (UK 415 Poland 231) | Primary | Cosinor analysisX2 | Seasonality observed for the total cohort (MOB: Dec). For sex subgroups, no significance for ♂.UK total: winter (Dec), OR 1.41, p=0.029.UK ♀: winter (Dec), OR 1.47, p=0.032.Poland total: winter (Jan), OR 1.5, p=0.04.Poland ♀: winter (Jan), OR 1.8, p=0.009. | 89 | |
| Sivalingam et al., 2018 | Denmark | The Danish National Patient Registry (DNPR), The Danish National Prescription Registry (DNPrR) | Case-control | GDAdults | 36,087 | Primary | Walter Elwood Test,Cox regression model (HR) | No | 89 | |
| Thvilum et al., 2017 | Denmark | The Danish National Patient Registry (DNPR), The Danish National Prescription Registry (DNPrR) | Case-control | HTAdults | 111,565 | Primary | Walter Elwood Test, Cox regression model (HR) | YesMOB: June (Walter test p=0.036),Individual risk: June (HR 1.04) (only for ♀), summer (HR 1.02) | 89 | |
| Valent et al., 2016 | Italy (Friul-Venezia Giulia) | Regional diabetes registry | Case registry | T1D0–18 y | 123 | Secondary | Lancaster's mid p exact test | YesSpring (May) and summer (July) | Higher regional T1D incidence.Peak month of diagnosis in August. | 56 |
Peak seasons are expressed according to the corresponding hemisphere (i.e., for the Northern hemisphere, autumn (Sept–Nov), winter (Dec–Feb), spring (March–May), summer (June–Aug); for Australia and New Zealand, spring comprises the months of Sept–Nov).
This study used data from previous publications in Europe: Sardinia,31 Sicily,32 Ireland,33 Berlin,34 Slovenia35 and Baden-Wuerttemberg36; in Israel37; and in New Zealand.38
♂: males; ♀: females; ↓: reduced; AAD: Addison's disease; Ab: antibodies; AITD: autoimmune thyroid disease; CI: confidence interval; GD: Graves’ disease; HR: Hazard ratio; HT: Hashimoto's thyroiditis; MOB: month of birth; OR: odds ratio; RR: relative risk; T1D: type 1 diabetes; TPOAb: anti-thyroperoxidase antibodies; UV: ultraviolet.
We used the “Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies” (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools)14 to assess the quality of the studies included. This tool consists of a 14-item questionnaire, which helps to critically reflect on the study's quality, beyond a mere numeric score, and obtain a “quality rate”. Details are shown in Supplementary Tables 1A and 1B. Selective descriptive and publication biases were eluded by an extensive search in multiple sources, including grey literature.
ResultsStudy characteristicsThe initial search retrieved 300 studies. After removing duplicates and performing the full text screening, we finally yielded 11 studies: 5 in patients with T1D, 5 in patients with AITD, 2 of which had HT exclusively, and 1 GD, and 1 for patients with AAD. Fig. 1 shows the PRISMA flow diagram for the selection of studies. Most studies included patients from Europe and USA, and only two studies were performed in individuals in the Southern hemisphere. Four studies included patients from different countries. The majority used regional or national registries as the main source of information. Control patients were taken from national registries of healthy individuals or healthy siblings. Table 1 summarises the results of the 11 studies selected.
PopulationThe revision included a total of 172,787 patients with AED: 18,169 with T1D (10.6%), 40,226 with GD (23.0%), 113,746 with HT (66.0%) and 646 patients with AAD (0.4%). Four studies included only adult patients,15–18 six studies also included patients under 18 years old,19–24 and one study did not specify patients’ ages.25
Study methods and quality assessmentAll studies retrospectively evaluated the seasonality in the MOB for AED: 2 case registries (18%) and 9 case-control studies (82%). Exploring this seasonality in the MOB was the primary objective for 9 studies, and the secondary objective for the remaining 2.21,23 Statistical analysis were performed by evaluating the annual rhythmicity for the MOB using cosine functions (5 studies, 45%), specific seasonality tests (Walter-Elwood test in 3 studies, 27%), linear regression models,21χ2 test,24 and Fisher's exact test.23
Mean quality score was 81.0±14.9% (range 51.2–100%), but four studies yielded a score below 70% (Supplementary Tables 1A and 1B).
Seasonal patternsAnalysis of the seasonality in the MOB in patients with endocrine autoimmune diseases is shown in Table 2. Eight studies (73%) found a clear seasonality pattern, but 2 did not. Sub-analysis for each disease found the following differences: all studies including HT patients found a seasonality for the MOB, the majority for spring and/or summer, whilst only one study with patients with GD15 observed a seasonality for spring. The majority of studies with T1D patients also found an increased seasonality in spring and summer. The sole study for AAD found an increased number of births in December.
Summary of the main findings regarding seasonality of the MOB according to the specific endocrine disease, sex, and the geographic location.
| Disease | Study (author, year) | Winter (Dec–Feb) | Spring (March–May) | Summer (June–Aug) | Autumn (Sept–Nov) | ||||
|---|---|---|---|---|---|---|---|---|---|
| ♂ | ♀ | ♂ | ♀ | ♂ | ♀ | ♂ | ♀ | ||
| T1D | Elliot et al., 2010 | – | – | – | – | ||||
| Laron et al., 2005 | – | New Zealanda | Europa (Sardinia, Sicily, Ireland, Slovenia) | Israel; USA (St Louis, Afro-Americans) | |||||
| Lewy et al., 2008 | (1) | Berlin | Sweden | Berlin | |||||
| Mikulecky et al., 2016 | Slovakia (adults) | Slovakia (teenagers) | Slovakia (teenagers) | Slovakia (2) | |||||
| Valent et al., 2016 | – | – | Italy | Italy | – | ||||
| GD | Hamilton et al., 2014b | – | – | – | – | ||||
| Krassas et al., 2007b | – | Greece | – | – | Greece | ||||
| Sivalingam et al., 2018 | – | – | – | – | |||||
| HT | Hamilton et al., 2014b | – | – | – | – | UK Denmark, Holland (3) | |||
| Krassas et al., 2007b | – | Greece | – | Greece | – | ||||
| Kyrgyos et al., 2018 | – | Greece (4) | – | – | |||||
| Thvilum et al., 2017 | – | – | Denmark (5) | – | |||||
| AAD | Pazderska et al., 2016 | UK, Poland (6) | – | – | – | ||||
Studies by Hamilton et al., and Krassas et al., are shown more than once because of their different results depending on the disease considered.
(1) Only in Berlin for the subgroup of males with high titers of auto-antibodies.
(2) Early autumn for T1D presenting in children, and late autumn for cases with later-onset.
(3) Only for women in the OXAGEN AITD Caucasian Family Collection cohort.
(4) Significant result for the study month-to-month and season-to-season, with peak incidence for March (OR 2.34), but not significant for the subgroup of women when sub analyzed by gender.
(5) Significant result for MOB, with a higher individual risk of developing HT in the month-to month and season-to-season study only for men, but not for women.
(6) When evaluating the cohorts separately, peak incidence for the MOB was only found for women in winter.
The study by Hamilton et al.16 found a trend for a seasonal pattern only for women in the cohort “OXAGEN AITD Caucasian Family Collection”. Most studies showed an increased risk for the months in spring (6 studies, 55%) and/or summer (6 studies, 55%); but 4 studies (36%) showed a peak for the MOB in autumn, and 3 (27%) for winter. One of these latter studies15 found a significant increase in the MOB for autumn and winter only for the subgroup of female patients with GD and HT, respectively; and Lewy et al.20 found an increase for the winter months only for the male patients in the Berlin cohort. Interestingly, some studies found a seasonality for the MOB for two seasons for the same population: Lewy et al.20 in spring and autumn for T1D in the Berlin cohort, Valent et al.23 in spring and summer in T1D in Italy, and Krassas et al.15 in spring and autumn for GD and winter for HT in Greece.
Factors that influence seasonalityNine studies explored the potential influence of sex on seasonality. Three studies did not find differences,18,21,22 while the remaining six did. Specifically, 4 studies16,17,24,25 observed a seasonality for the MOB in AED only in the subgroup of female patients, and Krassas et al.15 and Lewy et al.20 observed additional peaks in the subgroup of men (Table 2). In the study by Thylium et al.,17 the difference between sexes was only observed when evaluating the association month-by-month, but not season-by-season.
Two studies explored the potential association between the MOB and the age of onset of the disease. Mikulecky et al.22 found an increased peak of births in spring and early autumn for T1D presenting in children, and late autumn and winter for cases with later-onset. Conversely, Kyrgios et al.24 did not find significant differences.
The association of serum levels of autoantibodies at the time of disease diagnosis and the MOB was also explored. In this regard, Krassas et al.15 found seasonality in women with high-titre anti-thyroperoxidase antibodies. Lewy et al.,20 for their part, observed seasonality of the MOB only in patients with elevated anti-beta-cell antibodies at the time of T1D diagnosis.
Regarding environmental factors, Mikulecky et al.22 evaluated the monthly incidence of Coxsackie virus infections, which overlapped with the peak incidence of births during summer and autumn for children with T1D. In addition, two studies evaluated a potential seasonality in exposure to ultraviolet (UV) radiation and presumed vitamin D levels; in this regard, Elliot et al.21 found fewer cases of T1D in latitudes with a high UV radiation exposure, and subsequent enhanced induction of vitamin D synthesis, only in low-density population areas, whilst in highly populated areas, the incidence of T1D increased in relation to an increased exposure to UV radiation. Pazderska et al.25 did not find an association between the latitude and the seasonality in the MOB in patients with AAD.
DiscussionOverall, this systematic review suggests a seasonal pattern for an increased rate of births in spring and summer for patients who later developed AITD, T1D, and in winter for patients who developed AAD. Some studies found slightly different patterns, suggesting the influence of concomitant additional factors.
The seasonal pattern was more evident in women. Although autoimmune diseases are per se more frequent in women, several factors may contribute to this clearer seasonality of the MOB in women than in men. For instance, sexual hormones may have a role in triggering the immune response and loss of tolerance to self-antigens.26 In fact, oestrogens determine the cytokine profile, with a predominance for developing T helper type 1 (Th1) cell responses. Moreover, vitamin D levels may be lower in women,27 thus determining aberrant immune mechanisms.
Age was also associated with different seasonal patterns. Specifically, early age of onset of T1D was associated with an increased rate of births during the months of spring and summer, while later onsets occurred mostly in patients born during late autumn and winter.22 This could suggest that patients prone to developing T1D born during spring and summer exhibit a trend for an earlier onset and a more rapid disease progression.
It is interesting to comment on the potential role of geographical differences. In this regard, studies performed in high-density populations, such as Australia, Denver or Pittsburg, did not find rhythmicity in the MOB.19,21 However, the study by Laron et al.19 included different sets of populations, and they observed that only homogeneous populations exhibited seasonality. This could reflect the fact that factors common to all individuals would determine a seasonality of the MOB only in the specific setting of relatively similar individuals from a genetic and sociodemographic point of view, such as Ashkenazi Jews or Afro-Americans in St Louis, whilst in heterogeneous samples, the trend for a specific seasonality would be mitigated.
Seasonality was also different between AED. HT was the disease most represented across the literature, and was the one that most evidently showed a seasonal pattern. However, only the study by Krassas et al.15 described a seasonality for GD, which differed from that of HT. The weak seasonality could be explained by the lack of specific clinical or analytical data in the patients studied, which could have otherwise evidenced seasonality in certain subpopulations if further data had been evaluated, such as thyroid morphology or autoantibody titres.18
In addition, the different seasonality observed between HT and GD suggests differences in their potential triggers and their seasonal variation. In fact, AED are based on an immune aberration; thus, if a particular factor occurs during the foetal or perinatal period, which could, for its part, have a seasonal pattern, it may rebound later during lifetime, and impact a particular rhythmicity in the disease and the patients’ MOB. Viral infections may be an example of this seasonal issue. Indeed, several common pathogens have been associated to AITD and T1D, such as enterovirus, parvovirus B19, and coxsackie, amongst others.24,28 Besides, several genetic susceptibility loci in the innate immune system in patients who develop these diseases seem to increase the risk of acquiring these infections.20,25 Therefore, in utero exposure to endemic viral outbreaks during the months of autumn and winter in genetically susceptible individuals could explain the trend for an increased rate of births during spring and summer in the patients included in this review. A clear demonstration of this in-utero exposure is troublesome, but the fact that congenital rubella is truly associated with several immune aberrations and endocrine diseases29 could support this hypothesis.
In turn, UV radiation and vitamin D levels also exhibit a seasonal variation. In this setting, and given the immune-modulator properties of vitamin D, a reduced exposure during pregnancy to UV radiation and low vitamin D status during winter could entail an increased seasonal risk to the offspring developing AED.12 In fact, the pattern observed by Elliot et al.21 agrees with the hypothesis that higher levels of UV exposure either directly or indirectly (via enhanced vitamin D synthesis) provide an immunosuppressive effect that protects against the development of T1D, whilst lower levels of UV radiation during winter and lower sun exposure result in vitamin D storage depletion, and a loss of protective Th-1 immunosuppression.21 Conversely, for instance, if early life exposure to low-dose vitamin D from fortified food occurs, differences in the seasonal pattern decrease, as was observed in male patients with T1D in a Danish study.30
To our knowledge, this is the first time that the seasonality in the MOB in patients with three major AED with a high impact on patients’ health and quality of life has been systematically reviewed. We used a wide-ranging set of key words and searched four large well-recognised scientific databases, following the recommended PRISMA guidelines, and performed a well-established quality assessment. A large number of patients from different geographical regions were included, allowing a greater representation of the general population. Moreover, several aetiological factors were simultaneously considered, such as MOB, sex and age, which were evaluated using regression models, and thus overcoming potential confounding factors.
The limitations of the review are mainly related to the heterogeneous populations of the studies included, their different statistical methods, and their retrospective nature, which implied that patients already exhibited a predisposition to developing the AED, and thus, the seasonal pattern observed may not be applicable to the general non-susceptible population. The validity of this review could be determined by the potential biases and confounding factors of the studies included, such as the coexistence with other autoimmune diseases, nutritional factors, socioeconomic level or the seasonal variations in fertility.
In summary, our review finds an increased risk of developing AED for individuals born in spring and summer, presumably determined by the seasonality observed in several potentially influencing factors. This seasonality was more evident for HT and for women. The effect of seasonal factors during foetal development, reflected by seasonal birth rate variations, could lead to future aberrant autoimmune responses that would manifest later during life. Further long-term studies with larger homogeneous populations should be performed to confirm these hypotheses.
Authors’ contributionsARL and GC contributed to study conception and design, performed the initial review of articles, researched, analysed and interpreted data, wrote, reviewed and edited the manuscript. MM reviewed and edited the manuscript. All authors critically revised the final version of the manuscript and approved it for submission and subsequent potential publication.
Data availability statementThe data that support the findings of this study are available from the corresponding author upon reasonable request.
FundingThis study was not subject to any funding from public or private entities.
Conflicts of interestThe authors declare that there is no conflict of interest that could be perceived as directly or indirectly prejudicing the impartiality of the research reported.






![Flow chart depicting the main details on the screening process, with selection and exclusion reasons for the articles finally included in this systematic review, adapted following the indications in http://www.prisma-statement.org [Page 2021]. Flow chart depicting the main details on the screening process, with selection and exclusion reasons for the articles finally included in this systematic review, adapted following the indications in http://www.prisma-statement.org [Page 2021].](https://static.elsevier.es/multimedia/25300180/0000006900000010/v2_202301310826/S2530018022001810/v2_202301310826/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
