A review is made of the basic aspects of creatine/creatinine metabolism and the close relationship between creatinine and muscle mass, which makes the former a biochemical marker of the latter. Emphasis is placed on the current prognostic value of both the low urinary excretion of creatinine and low serum creatinine levels in different clinical settings in which sarcopenia probably plays a significant role in morbidity and mortality.
Se revisan los aspectos básicos del metabolismo de la creatina/creatinina y la íntima relación entre la creatinina y la masa muscular, lo que la convierte en un marcador bioquímico de ésta. Se hace énfasis en la utilidad pronóstica actual, tanto de la baja excreción urinaria de creatinina como de los niveles bajos de creatinina sérica, en diferentes contextos clínicos en los que la sarcopenia probablemente desempeña un papel importante en la morbilidad y mortalidad.
When clinicians interpret creatinine levels, they are usually thinking about kidney function, and forget that creatinine also reports on the status of muscle mass (MM). The quantification of MM is essential in addressing conditions as common as malnutrition, primary or secondary sarcopenia, sarcopenic obesity, or type 2 diabetes mellitus (T2DM). The present study reviews the clinical significance of low creatinine levels, a subject that usually draws little attention outside Nutrition Units, but which has recently shown important epidemiological and prognostic value in multiple settings.
Historical referenceIn 1832, Chevreul1 identified an organic substance in meat juice, which he referred to as creatine (from the Greek kreatos, meat). He also found that heating creatine in the presence of hydrochloric acid transformed it into another substance that crystallized differently. In 1847, Liebig2 established the formula for creatine (C4H9N3O2) and called the substance derived from it “creatinine”, which he identified as “anhydrous” creatine (C4H7N3O).
In 1904, Otto Folin,3 by perfecting the Jaffe method,4 was able to measure creatinine in blood and urine. This author noted that creatinine excretion in urine is relatively stable in each individual over time (though with great variability between individuals) and also stated that “the main factor determining the amount of creatinine excreted appears to be the weight of the individual, provided protein intake is stable”. However, he also found that the more obese the patient, the less the amount of creatinine excreted per kg body weight, thus concluding that the amount of creatinine excreted depends on the mass of “active protoplasmic tissues”.5 Folin thus established two concepts that still apply today, though with important nuances: 1) the usefulness of measuring creatinine in 24-h urine to assess whether it has been collected in its entirety; and 2) the initial proposal of what would later be called a “creatinine coefficient”, consisting of dividing 24-h urine creatinine by the weight of the individual, expressing the ratio between urinary creatinine (the almost exclusively muscular origin of which was not known at that time) and body weight. In healthy individuals, this ratio was then considered to remain more or less constant.
In 1907, Spriggs6 found various types of muscle atrophy to be associated with low urine creatinine levels. In 1913, Myers and Fine7 demonstrated that creatinine excretion in urine is directly proportional to the creatine content in muscle. In 1919, Bürger8 estimated that a urinary excretion of 1 g creatinine/day in humans corresponds to approximately 22.9 kg of skeletal muscle, based on the notion that a male weighing 63.1 kg excretes 1.36 g creatinine/day and has 25.4 kg of muscle, as shown by post-mortem studies. In later studies, this figure moved a little up or down, depending on the method used to measure creatinine or MM, and on other factors that will be analyzed below.9–14 Finally, the Baltimore Longitudinal Study on Ageing15 found basal O2 consumption to decrease with age from the age of 45 years, in parallel to MM loss as assessed by 24-h urinary excretion (24hUECr).
The first study to directly compare urinary creatinine excretion with the amount of MM measured by full body computed tomography (CT) was published in 1996 by Wang et al.16 These authors confirmed the existence of a strong correlation between both parameters in 12 healthy and physically active adult males on a meatless diet, and from whom complete 24-h urine samples were obtained. In these individuals, the relationship between MM and 24hUECr was seen to fit the model MM = 21.8 × 24hUECr, which is very consistent with the classical data. In addition, the Forbes17 group showed 24hUECr to be positively correlated to the cross-sectional areas of the arm and thigh muscles (r = 0.85 and 0.88, respectively), in both 15 healthy young men and 9 healthy young women (under 32 years of age) and in 23 men and 16 women over 60 years of age. In 1999, Proctor et al.18 compared the usefulness of different methods for evaluating MM (or lean mass) in young, middle-aged and elderly men and women, and repeated the evaluation three months later. These authors found a strong correlation between 24hUECr and MM as measured by DEXA (dual-energy X-ray absorptiometry), with r = 0.8 in all groups. They also confirmed a strong inverse correlation between 24hUECr and age in men and women. The main problem with 24hUECr was its low reproducibility on repeating the evaluation (17.7% versus 4.1% for DXA).
In view of the difficulties of securing adequate 24-h urine collection, particularly in the clinical setting, as the techniques for measuring serum creatinine (SCr) improved, interest developed in the possibility of measuring SCr to assess MM. Finally, a significant correlation between both 24hUECr and SCr and MM was established, as assessed by different methods,19–22 including DEXA21 or electrical bioimpedance (EBI),22 in healthy male and female subjects with variable activity levels.
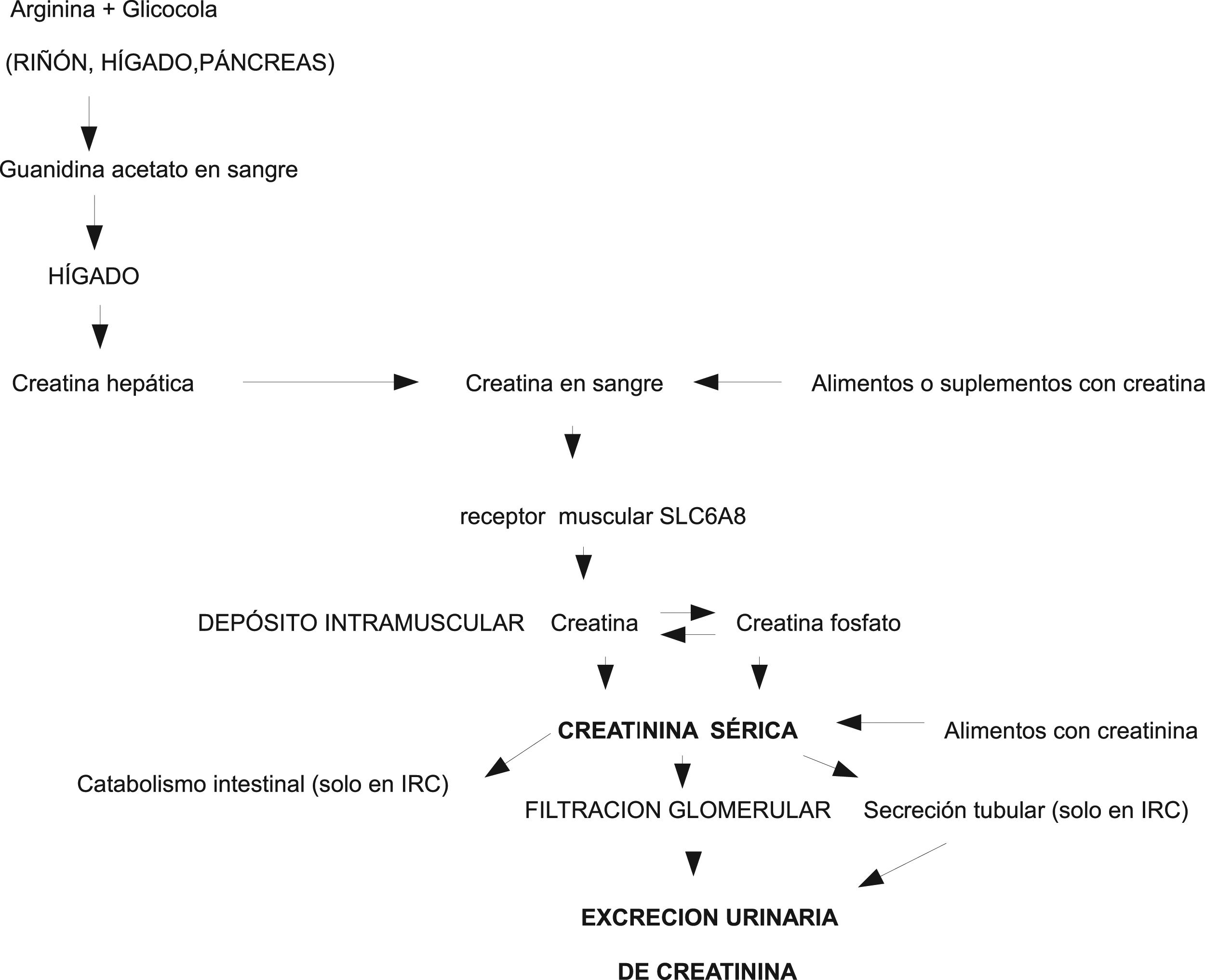
Physiopathology of creatine/creatinineCreatinine is a small molecule produced by dehydration with the non-enzymatic spontaneous cyclization of creatine and phosphocreatine in muscle. In the in vivo setting, the conversion of creatine into creatinine is an irreversible and constant process.23 A 70 kg male is estimated to contain approximately 28 kg of muscle tissue and 120 g of total creatine (creatine plus phosphocreatine). Of this amount, approximately 2 g/day is converted into creatinine and must be replaced through endogenous synthesis or exogenous supply. The ingestion of 500 g of meat or fish alone is sufficient to cover the daily requirements of creatine.23 The endogenous biosynthesis of creatine is mediated enzymatically in the kidney and liver.23,24 Creatine is released from the liver into the blood, and a membrane-specific transporter (SLC6A8) mediates its entry to the muscle where it is stored, used and degraded, but not synthesized. Creatine is located in the sarcomere. It does not appear in non-contractile elements, and is therefore independent of the water, fibrotic or adipose component that may accumulate within diseased muscle23 and complicate the quantification of MM using DEXA or CAT (Fig. 1).
Creatinine permeates through cell membranes, freely diffuses from cells into the blood, is not bound to plasma proteins, is not stored or metabolized in any tissue, and is rapidly excreted by the kidneys through direct glomerular filtration. Although it was initially thought that creatinine was not excreted or reabsorbed in the renal tubules, it is now known that a variable part is excreted into the urine from the tubules, and that such excretion increases as glomerular filtration deteriorates.25 Since the non-enzymatic transformation of creatine into creatinine is virtually constant, and more than 90% of the total body creatine is found in muscles, 24hUECr is regarded as a good marker of total body MM.13
However, many factors can modify the results of creatinine measurement in both blood and urine. In addition to the measurement technique used, the most important factors are diet, age, gender, race, physical exercise, renal failure, certain drugs and diseases (particularly neuromuscular disorders), and especially the essential need for complete 24-h urine collection. This is the limiting factor in many cases, particularly in people with involuntary urinary losses, incomplete bladder emptying, or in those who fail to fully collaborate in the urine collection process.25 Diet influences creatinine excretion via at least three mechanisms24: 1) dietary proteins are the main source of amino acids needed for the endogenous production of creatine; 2) dietary creatine can range markedly from nearly zero in strict vegetarians to several grams per day in individuals taking creatine supplements, while the average “American” diet of 200 g of meat is capable of replacing one-third to one-half of the normal creatinine urinary losses; and 3) dietary creatinine, also of animal origin, supplies 37−160 mg/day, depending on how it has been cooked. Diet is therefore an important determining factor of variability in creatinine excretion. The age, gender, race and physical activity of the individual condition the amount of MM, which in turn conditions 24hUECr. Intense exercise temporarily increases urine creatinine levels. Severe acute inflammatory conditions associated with fever or trauma and some neuromuscular diseases likewise increase creatinine excretion when there is excessive muscle catabolism. Certain drugs (trimethoprim, cimetidine, imatinib, etc.) transiently decrease 24hUECr and increase SCr, without this implying renal failure.25 In order to adequately assess the blood and urine creatinine results, careful control is required of all the factors indicated in Table 1, as well as of the method used to measure creatinine (modified Jaffe, enzymatic, HPLC/LC, etc.).
Situations in which serum creatinine is modified.
| Situations in which serum creatinine decreases |
|---|
| Pre-renal cause: Volume expansion (syndrome of inappropriate ADH secretion, pregnancy, acute volume overload, etc.) |
| Renal cause: Glomerular hyperfiltration |
| Muscle cause: Low muscle mass of any cause (primary sarcopenia of the elderly, sarcopenia secondary to chronic diseases, malnutrition, lack of physical activity, limb amputation, neuromuscular diseases [paraplegia, hemiplegia, amyotrophic lateral sclerosis, various myopathic conditions]) |
| Hepatic cause: Severe liver failure |
| Type of diet: Strict vegetarian diet |
| Situations in which serum creatinine increases |
|---|
| Pre-renal cause: Volume depletion |
| Renal cause: Acute or chronic renal failure, inhibitors of the tubular excretion of creatinine (cimetidine, trimethoprim, dronedarone, probenecid, salicylates, dolutegravir, imatinib, etc.) |
| Muscle cause: Muscle hypertrophy (genetic, hormonal, chronic physical exercise, etc.) or acute muscle damage (excessive acute physical exercise, rhabdomyolysis from any cause, etc.) |
| Type of diet: Consumption of large amounts of cooked meat or fish, intake of creatine supplements |
Metter et al.26 were the first to show 24hUECr to be lower in men who died than in those who survived in the Baltimore Longitudinal Study of Aging after 25 years of follow-up, though 24hUECr was not the primary study endpoint (the authors actually studied the prognostic value of muscle strength).
The PREVEND study (Groningen prospective general population cohort),27 published in 2009 and which monitored 8092 subjects for 7.5 years, showed low 24hUECr in women to be associated with a clear increase in the risk of major adverse cardiovascular events (MACE) and an increased risk of overall mortality regardless of factors such as age, race, smoking, a previous history of cardiovascular disease, insulin resistance or low-grade inflammation assessed by high-sensitivity C-reactive protein (hsCRP). A significant increase in mortality risk and an almost significant increase in MACE were also observed in men as 24hUECr decreased. These authors were the first to suggest that low 24hUECr levels, indicative of calorie-protein malnutrition or subclinical sarcopenia in the general population, could be an independent marker of serious chronic diseases such as atherosclerosis, though other factors could also be postulated (diet, sedentary lifestyle, type of life, etc.).
The Heart and Soul Study28 prospectively followed-up on 903 patients with stable coronary artery disease at the time of enrolment, for a period of 6 years. The patients in the lowest 24hUECr tertile showed a two-fold increase in mortality risk compared with those in the highest tertile after multiple adjustments for age, gender, race, the glomerular filtration rate (estimated by cystatin C), the body mass index (BMI), traditional cardiovascular risk factors, and hsCRP levels. A later study of this same cohort29 found that low levels of both 24hUECr and the BMI were associated with increased all-cause mortality, but that low 24hUECr levels alone did not appear to explain the increase in mortality induced by a low BMI.
The Groningen group30 also showed that patients with chronic systolic heart failure and low 24hUECr had smaller body dimensions and more severe heart failure, and low 24hUECr was moreover also predictive of a poor cardiac outcome. It has also been reported that patients in the lowest 24hUECr tertile upon admission due to stroke have a poorer prognosis than patients in the highest tertile.31
In 2013, Sinkeler et al.32 published a post hoc analysis of the combined databases of the RENAAL and IDNT trials (3228 patients followed-up on for an average of 3.5 years) in which low 24hUECr was found to be associated with an increased rate of all-cause mortality in patients with T2DM and diabetic nephropathy. Subsequent multicenter studies33–35 confirmed these data. The Groningen group found low 24hUECr to be associated with a self-diagnosis of frailty in patients with chronic renal failure.36 This same group also found 24hUECr to be lower in renal transplant patients as compared to a control group with the same renal function, and this was moreover correlated to muscle strength as measured by a grip strength dynamometer.37 Finally, low 24hUECr has been shown to be an independent predictor of mortality and graft failure in both kidney38 and liver transplant patients.39
Since 1993, some patients admitted to intensive care units (ICUs) have been found to have low 24hUECr, without this being due to inadequate urine collection or renal failure.40 The phenomenon was seen in up to one third of all patients, particularly in women and the elderly. In 2018, the Groningen group41 published the results of 6151 patients in whom 24hUECr was measured during the first three days of ICU admission, with the exclusion of grade 3 or higher renal failure. In this clinical setting, low 24hUECr was also associated with increased short- and long-term mortality, independently of age, gender, renal function or causal disease.
Prognostic value of low serum creatinine (SCr)In the same way that low 24hUECr is associated with a poor prognosis in many situations, low SCr values are likewise associated with a poor prognosis, though it took longer for this fact to become recognized.
There are no specific data on the prognostic relevance of low SCr in the general population, though in 2017 Thongprayoon et al.42 analyzed 73,994 patients admitted to the Mayo Clinic between 2011 and 2013, in whom SCr upon admission and its clinical course had been measured. The relationship between baseline SCr and in-hospital mortality showed a U-shaped distribution, and after adjusting for age, gender, race, principal diagnosis and comorbidities, a baseline SCr < 0.4 mg/dl was seen to increase the mortality risk, even compared with those patients with SCr > 1.5 mg/dl.
The first study relating low SCr to T2DM was published by Harita et al. in 2009.43 These authors prospectively followed-up on 8570 non-diabetic Japanese men between 40–55 years of age over a period of four years. The subjects with lower baseline SCr levels (0.4−0.6 mg/dl) had a greater risk of developing T2DM after multiple adjustments for confounding factors (including the BMI) as compared to those with SCr 0.71−0.8 mg/dl. This was attributed to the fact that the group with lower SCr levels comprised patients with a lesser MM, though the latter was not measured directly. In this regard, several Asian studies44,45 have established that in non-diabetic adults, low MM measured by electrical bioimpedance (EBI) predicts progression to T2DM, regardless of the presence of obesity. However, these studies did not measure SCr or 24hUECr.
Kashima et al.46 studied 9667 non-diabetic and non-hypertensive Japanese individuals (men and women) with normal basal SCr followed-up on for 5.6 years. Men with SCr < 0.7 mg/dl and women with SCr < 0.5 mg/dl were seen to be at a greater risk of developing T2DM than those with higher levels. Another study involving 3313 Japanese men aged 30–55 years without diabetes and monitored during 6.7 years47 found lower basal SCr (<0.7 mg/dl) versus SCr 0.9–1.1 mg/dl to imply a greater risk of developing both T2DM and impaired fasting blood glucose (IFG). Although adjustments were made for age, the BMI, alcohol intake, physical activity, and a family history of T2DM, no measurements of MM were made. Three very recent studies in Asian individuals48–50 have again confirmed that low SCr predicts a progression from normal conditions to IFG and T2DM.
However, a prospective study of 2676 Korean men and women followed-up on for 4–5 years51 found no differences in basal SCr between those who did or did not develop T2DM. Nevertheless, a decrease in SCr over time was seen to be associated with the occurrence of T2DM after adjusting for the usual factors. This study showed SCr to be positively correlated to MM as measured by EBI. Of note in relation to the risk of T2DM and IFG is the lack of prospective studies measuring SCr and MM in European or sub-Saharan populations.
Goel et al.52 studied the course of 4394 patients subjected to percutaneous coronary revascularization over a period of 4.2 years and identified a subgroup with a normal BMI and low SCr (<0.7 mg/dl) in which both cardiovascular and all-cause mortality were significantly elevated compared to those individuals with normal SCr (0.71−0.97 mg/dl).
Huh et al.,53 in a group of 8648 Korean individuals (including more than 4000 postmenopausal women) with normal renal function, found SCr to be positively correlated with appendicular MM and the T-score in the femur and lumbar region, again underlining the notion that low SCr indicates low MM and is also a predictor of the risk of densitometric osteoporosis (fractures were not assessed).
Cartin-Ceba et al.,54 as early as 2007, had found that low SCr upon admission among over 11,000 patients in the ICU of the Mayo Clinic predicted mortality regardless of the BMI. These authors likewise speculated that low SCr may be indicative of low MM or malnutrition, thus defining low SCr as a marker of lesser capacity to respond to the critical situation. However, low SCr could also be attributable to hemodilution due to fluid overload or increased renal clearance syndrome in critically ill patients. More detailed studies are needed in this regard.
In 2016, Udy et al.,55 in a monumental retrospective multicenter study that included more than one million patients, confirmed that low SCr at ICU admission is associated with an increased risk of in-hospital mortality, independently of other confounding factors. However, these authors likewise did not measure MM directly; the relationship with MM therefore could not be studied. Kang et al.56 also demonstrated that a decrease in SCr during the ICU stay is predictive of increased mortality in these patients.
ConclusionsThere is robust epidemiological evidence in the reviewed clinical settings (the general population, the elderly, coronary disease, diabetic nephropathy, patients in hospital wards or intensive care, transplant patients, etc.) that both low 24hUECr and low SCr suggest a poor outcome in terms of morbidity (increased cardiovascular risk, worsening heart failure, progression towards T2DM, the existence of osteoporosis and frailty, poorer graft evolution, etc.) and global mortality. Citing the precise and prophetic editorial published by Kalantari and Bolton,57 we can affirm that “there is good reason for measuring 24hUECr, beyond the assessment of renal function”. This measurement is inexpensive and easy to perform in cooperative patients who are in hospital or catheterized - such as those admitted to intensive care - but is difficult to generalize to patients. However, it seems advisable to pay more attention to all patients with low SCr values, which are identifiable and available in all clinical histories, but which often go unnoticed in routine evaluations. In most studies, the threshold beyond which risk clearly increases is 24hUECr < 700 mg/day in males (600 in females) or SCr < 0.6 mg/dl in males (0.5 in females), though these figures depend on the context and on the multiple factors described in Table 1. No official recommendations are therefore available in this regard. The most likely cause of low creatinine in these situations is sarcopenia (whether primary or secondary), taking into account the good correlation between creatinine and MM in healthy individuals11–22 and in different disease settings,51–53,58 though further prospective studies are needed involving the simultaneous determination of creatinine and MM. In any case, in patients with low creatinine, it is advisable to perform a nutritional assessment and re-evaluate diet and physical activity, since in potential we may improve the prognosis, though this should still be confirmed through well-planned clinical trials. Lastly, there are other situations in which low creatinine also appears to be a useful marker (cancer-related cachexia, patients on dialysis, chronic liver failure, chronic lung disease, etc.); however, the great complexity inherent to these processes means that many more studies are needed. In any case, in patients with low creatinine we should think of possible sarcopenia, and a nutritional assessment should be made, with re-evaluation of both diet and physical activity.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Amado Diago CA, Amado Señaris JA. ¿Debemos prestar más atención a la creatinina baja? Endocrinol Diabetes Nutr. 2020. https://doi.org/10.1016/j.endinu.2019.12.008