Vascular endothelial growth factor (VEGF) plays an essential role in development of diabetic macular edema (DME). While there is evidence suggesting that silymarin, a flavonoid extracted from Silybum marianum, could be useful for prevention and treatment of diabetic nephropathy, no studies have been conducted in diabetic retinopathy (DR). The aim of this study was to assess the effect of silymarin on disruption of inner blood retinal barrier (BRB), the primary cause of DME.
Materials and methodsHuman retinal endothelial cells (HRECs) were cultured under standard (5.5mM D-glucose) and diabetogenic conditions (25mM D-glucose and 25mM D-glucose + recombinant vascular endothelial growth factor [rVEGF, 25mg/mL]). To assess cell viability, three concentrations of silymarin were tested (2, 4 and 10μg/mL). The effect of silymarin on HREC disruption was determined using a dextran (70kD) permeability asssay.
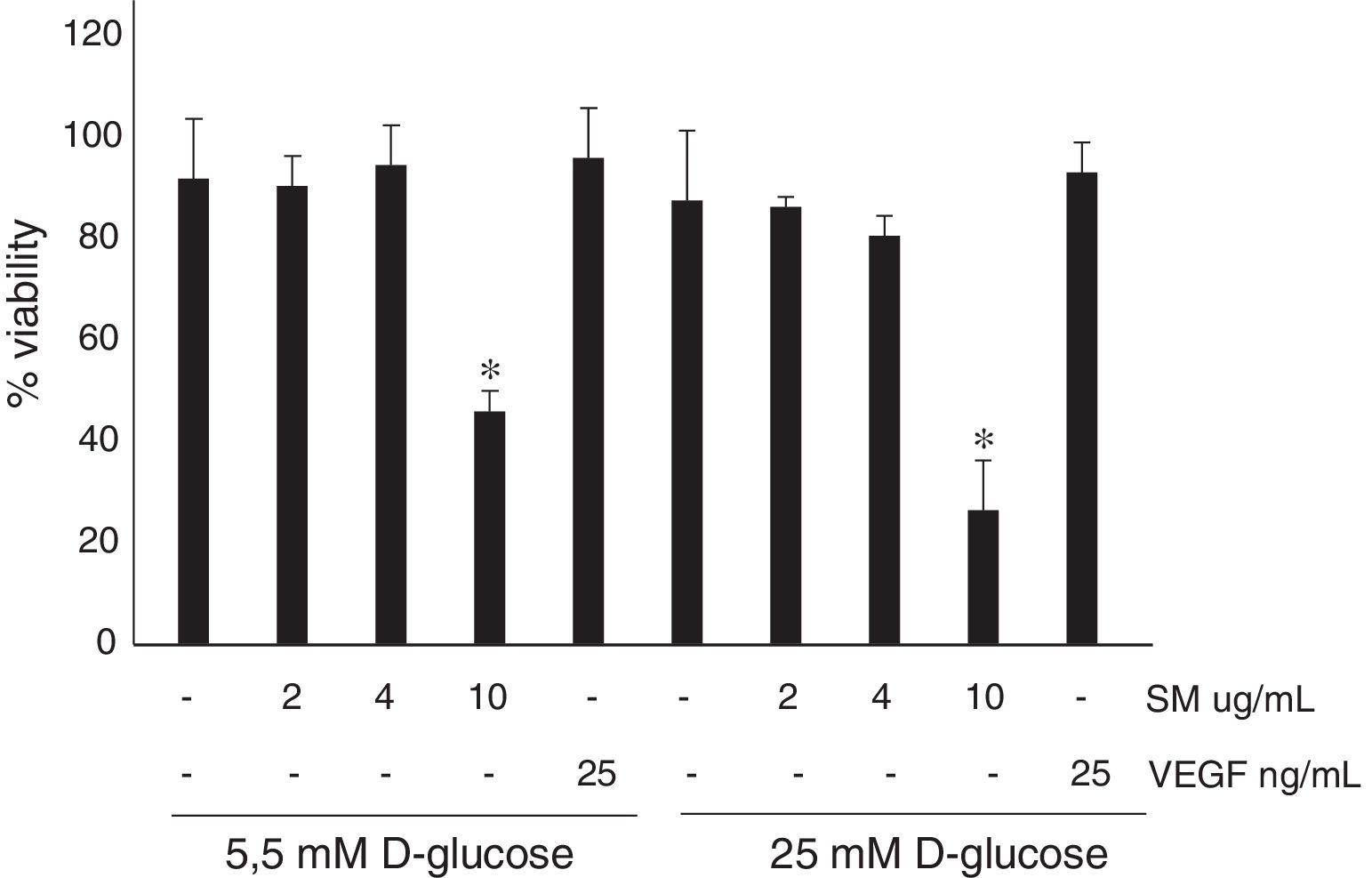
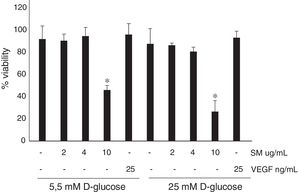
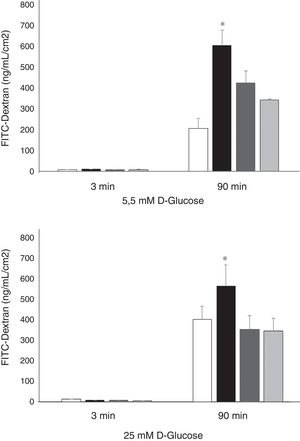
ResultsNo differences were found in the viability of HRECs treated with 2 or 4μg/mL of silymarin as compared to untreated cells, but viability significantly decreased after using 10μg/mL. The concentration of 4 μg/mL was therefore selected. Silymarin (4μg/mL) caused a significant decrease in VEGF-induced permeability in both media with 5.5nM (422±58 vs. 600±72 ng/mL/cm2; p<0.03) and 25nM of D-glucose (354 ± 28 vs. 567 ± 102 ng/mL/cm2; p<0.04).
DiscussionOur results show that silymarin is effective for preventing hyperpermeability induced by diabetic conditions in HRECs. Further studies are needed to assess whether silymarin could be useful to treat DME.
El Vascular endothelial growth factor (VEGF) juega un papel esencial en el desarrollo del edema macular diabético (EMD). Existe evidencia que indica que el uso de la silimarina, extracto flavonoide del Silybum marianum, podría ser útil en la prevención y el tratamiento de la nefropatía diabética pero no se dispone de datos en retinopatía diabética (RD). El objetivo del estudio es evaluar el efecto de la silimarina sobre la disrupción de la barrera hematorretininana, que es la causa primaria del EMD.
Material y métodosCélulas endoteliales de retina humana (HRECs) se cultivaron en condiciones estándar (5.5mM de D-glucosa) y en condiciones suprafisiológicas de glucosa (25mM de D-glucosa y 25mM de D-glucosa + VEGF 25mg/dl). Para evaluar la viabilidad de las células se probaron 3 concentraciones de silimarina (2, 4 y 10μg/ml). El efecto de la silimarina sobre la disrupción de las HRECs se determinó mediante análisis de permeabilidad a dextrano (70kD).
ResultadosNo se observaron diferencias en la viabilidad de las HRECs tratadas con 2 o 4μg/ml de silimarina en comparación con las células no tratadas, pero se observó una reducción de la viabilidad con la concentración de 10μg/ml. Por consiguiente, se seleccionó la concentración de 4μg/ml de silimarina. La silimarina (4μg/ml) produjo un descenso significativo de la permeabilidad inducida por VEGF tanto en medio con 5.5mM de D-glucosa (422 ±58 vs. 600 ±72 ng/ml/cm2; p<0.03) como en medio con 25mM de D-glucosa (354±28 vs. 567±102 ng/ml/cm2; p<0.04).
DiscusiónNuestros resultados demuestran que la silimarina es efectiva para prevenir la hiperpermeabilidad inducida por condiciones suprafisiológicas de glucosa en HRECs. Son necesarios más estudios para evaluar si la silimarina podría ser útil para el tratamiento del EMD.
Diabetic retinopathy (DR) remains the leading cause for vision loss in developed countries.1 DR is the most frequent microvascular complication, the prevalence of which increases with the duration of diabetes, with an overall rate of up to 30% and a high risk of severe visual impairment in 10% of subjects.2,3 Diabetic macular edema (DME) is more frequent in type 2 diabetes, occurs in approximately 7.5% of diabetic patients, and is the main cause of vision loss in working-age adults in industrialized countries.4,5
Vascular endothelial growth factor (VEGF) is a well-known pathogenic factor for the disruption of the blood retinal-barrier (BRB) and together with proinflammatory cytokines play a key role in the development of DME.6,7 For this reason, the intravitreal injection of anti-VEGF agents such as ranibizumab, bevacizumab and aflibercept represents the first-line therapy for DME involving the central macula.8,9
Silymarin is a flavonoid extracted from Silybum marianum (Milk thistle), which contains seven major components: taxifolin, silychristin, silydianin, silybin A, silybin B, isosilybin A and isolilybin B.10 Silymarin has mainly been used to treat liver diseases due to its anti-oxidant, anti-fibrotic, and anti-inflammatory properties.11,12 In addition, recent experimental evidence suggests that silymarin has additional effects, which could be useful for prevention and treatment of diabetic complications.13–15 In this regard, silymarin is associated with an anti-glycation effect, inhibits aldose reductase, reduces liperperoxidation, and is a partial agonist of peroxixome proliferator-activated receptor γ (PPARγ).16,17 Notably, silymarin reduces urinary excretion of albumin, TNF-α, and malondialdehyde (MDA) in patients with diabetic nephropathy.18
Lin et al.,19 reported that silybin, a main component of silymarin inhibited VEGF secretion induced by hypoxia in retinal pigment epithelial (RPE) cells, and prevented VEGF- and VEGF plus hypoxia-induced retinal edema. In addition, they also provide evidence that silybin prevented neovascularization in a rat model of age-related macular degeneration (AMD).19 Zhang et al.,20 reported that silybin treatment significantly prevented the development of obliterated retinal capillaries in diabetic rats. All these findings points to silymarin as a potential therapeutic approach for VEGF induced hyperpermeability condition such as DR. However, the effect of silymarin on the vascular leakage induced by diabetes has not been previously reported.
On this basis, the main aim of the present study is to evaluate the effect of silymarin on VEGF induced hyperpermeability in human retinal endothelial cells (HREC) under standard and high glucose conditions.
Material and methodsCell culturesHuman retinal endothelial cells (HRECs) were obtained from a vial of cryopreserved cells purchased from Innoprot (Vizcay, Spain). The supplier, has isolated cells from human retinal tissue of cadaveric eyes digested with 0.1mg/mL collagenase type I at 37°C for 1 hour. Then, endothelial cells were finally selected with CD31 antibody-coated magnetic beads (DynaBeads; Dynal, Oslo, Norway). HRECs were thawed in the laboratory and cultured in endothelial basal medium (EBM) containing, 5,5mM D-glucose, 5% FBS (Fetal Bovine Serum), 100 U/mL penicillin, 100μg/mL streptomycin and ECG (Endothelial Cell Growth Supplement) supplement (Innoprot, Vizcay, Spain). Human fibronectin (MerckMillipore, Madrid, Spain) at 5μg/mL was used for cell attachment. Endothelial medium was changed each 3 days. Cells at passages 2-3 were used for the experiments. For experimentation, HRECs were grown to confluence and then cell culture media was changed to medium supplemented with 1% FBS for 24h and then exposed to different treatments. Recombinant human Vascular Endothelial growth factor (rVEGF165) was purchased from R&D systems (Vitro, Madrid, Spain). Silymarin was purchased from Sigma-Aldric (Madrid, Spain). 3-(4,5-dimethylthiazol- 2-yl)-2,5-diphenyl tetrazolium bromide (MTT) were obtained from Sigma-Aldrich (Madrid, Spain).
Cell viability and proliferationCell counting and MTT assay were performed in HRECs to assess the viability of cells treated with silymarin in order to select the appropriate concentration of silymarin. Briefly, HRECs, 1,5 × 103 cells/well were seeded into a 96-well plate. Cells were cultured until confluence (48h) and then arrested by serum deprivation (1%) for 24h. Then, cells were treated with Silymarin (2, 4 and 10 ug/mL) or rVEGF165 (25 ng/mL) for 48hours. Cells were stained with DAPI and photographs were taken (20x) in a fluorescent inverted microscop (Olympus iX71 with V-RFL-T Olympus). Cell nuclei were count with the help of Image J software in three different fields for each condition. The experiments were repeated three times. In addition, the MTT assay (Sigma, Madrid, Spain) was used in order to evaluate the viability of cells. Briefly, 10μl of 5mg/ml MTT in PBS was added to each well after the treatments and incubated an additional 1h at 37°C. The medium was removed and the formazan granules obtained were dissolved in 100% dimethyl sulfoxide (DMSO) and absorbance was detected at 562nm with an ELISA plate reader (ELx800. Bio-Tek Instruments, VT, USA). This assay permitted us to rule out a potential bias in the results due to changes in cell proliferation among the different conditions.
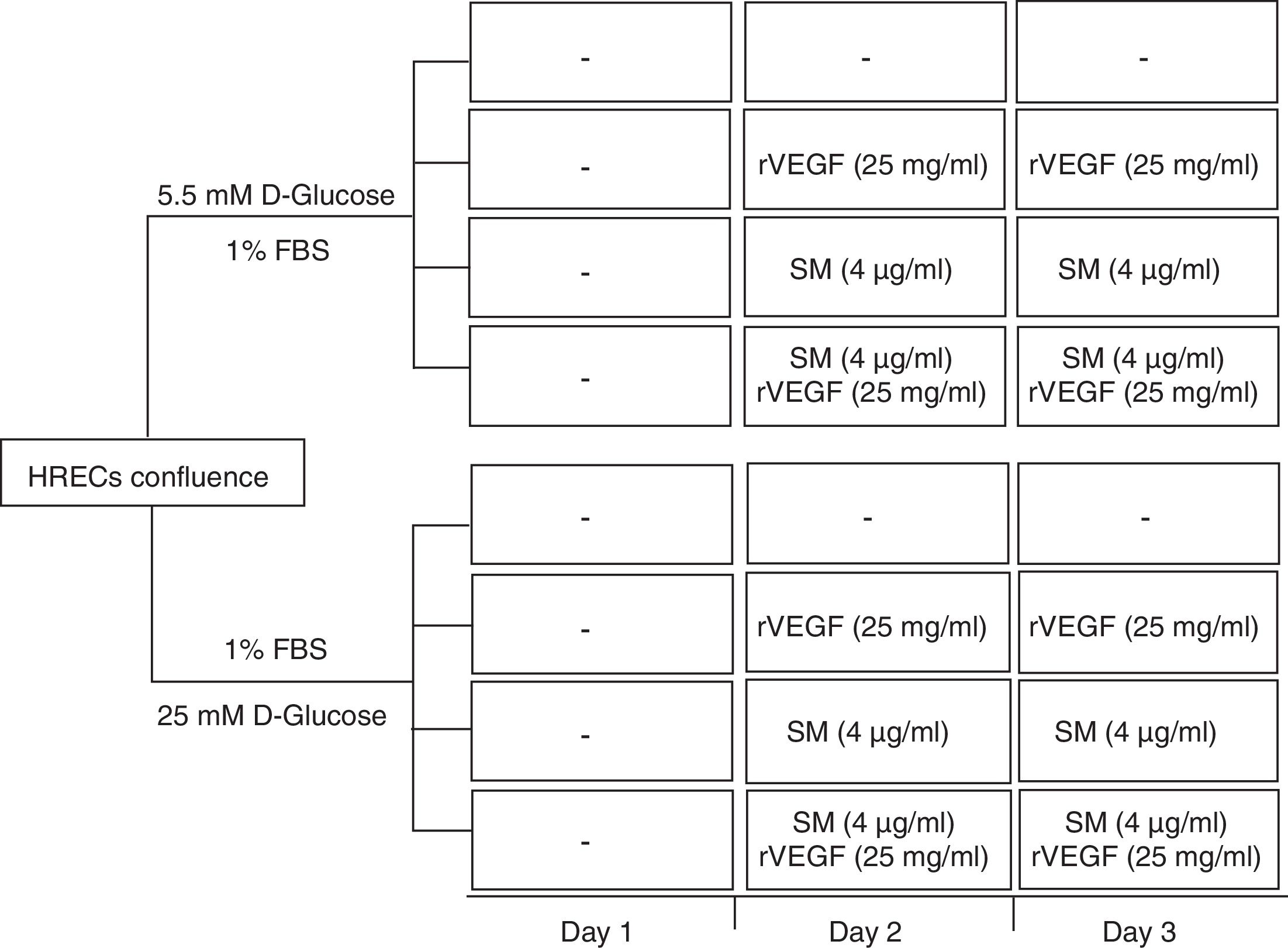
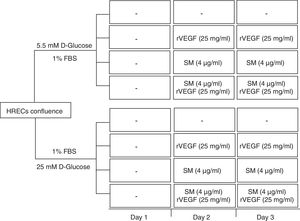
Measurement of HREC permeabilityPermeability in HREC monolayers was obtained on permeable supports at 1,2 x 105 cells/well (24 wells, PCF filters, MerkMillipore, Madrid, Spain). Inserts were incubated for 48hours at 37°C in 5% CO2-air to form the monolayer. At the end, medium was serum depleted 24h before to proceed with the treatments. The lower chamber was filled with 600μL of complete EBM medium and the upper chamber with 100μL of cell suspension in serum depleted (1%). Treatment conditions included: 5,5 or 25mM D-glucose, Silymarin (4 ug/mL) with or without rVEGF165 (25 ng/mL) (Figure 1). To detect changes in the permeability, 100μg/ ml of fluorescent FITC-DEXTRAN (70 KDa) (Sigma, Madrid, Spain), was added to the upper side of the insert. Aliquots of 200μL from the basal compartment were read in a SpectraMax Gemini (Molecular Devices, Sunnyvale, CA) at a wavelength of excitation/emission 485/528nm every 30min. Finally, the concentration of dextran was determined by extrapolation of the fluorescence reads in a standard curve. Each condition was tested in triplicate.
StatisticsData are presented as means ± SD. Comparisons of continuous variables were performed using ANOVA and Student's t test with SPSS software (SPSS Inc. Released 2009. PASW Statistics for Windows, Version 18.0 Chicago: SPSS Inc.). The statistical significance level was set at p <0.05.
ResultsNo significant differences were found in the viability in HREC treated with 2 or 4μg/ml of silymarin in comparison with untreated cells (Figure 2). By contrast, in HREC treated with 10μg/ml of silymarin a significant reduction of viability was observed. Silymarin at the high concentration tested (10μg/ml) decreases cell viability, and therefore to evaluate its effect on BRB permeability the concentration of 4μg/ml was used.
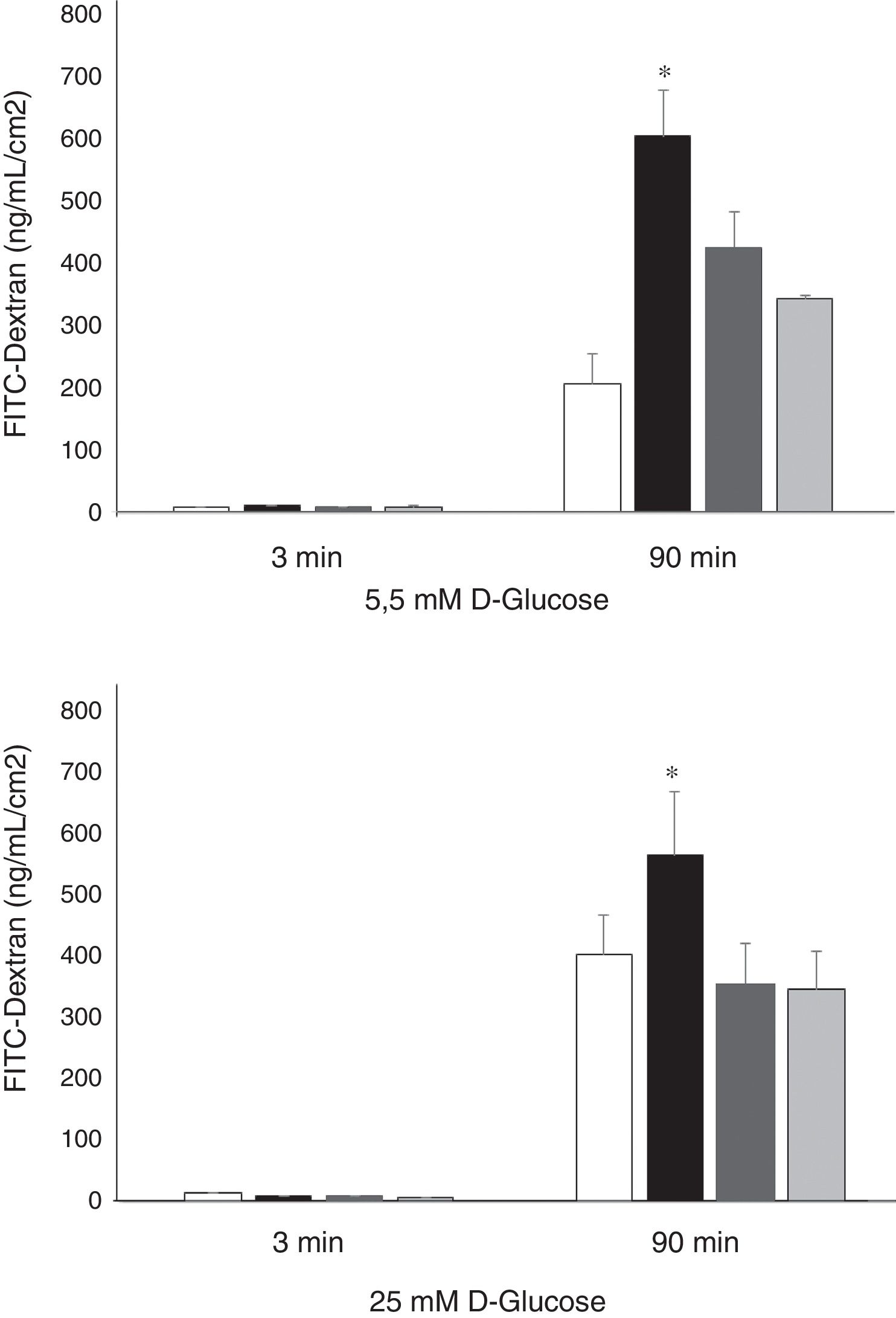
Permeability of HREC monolayers were higher in high glucose (25nM) conditions in comparison with normal glucose conditions (5.5mM) (403±140 vs. 206±26; p<0.008) (Figure 3). rVEGF165 (25 ng/mL) significantly increased the permeability of 70 kDa-Dextran through HRECs monolayers cultured under both, normal (600±72 vs. 206±47 ng/mL/cm2; p<0.02) and high glucose conditions (567±102 vs. 403±64 ng/mL/cm2; p<0.05). Silymarin (4 ug/mL) produced a significant decrease of VEGF induced permeability under either 5.5mM (422±58 vs. 600±72 ng/mL/cm2; p<0.03) or 25mM of D-Glucose (354 ± 28 vs. 567 ± 102 ng/mL/cm2; p<0.04) (Figure 3).
Results of 70 kDa dextran permeability of Silymarin in HREC cells cultured under 5,5 mmol/L D-glucose or 25 mmol/L D-glucose. HRECs cultured in monolayers were treated with two doses of Silymarin (4 ug/mL) with/without rVEGF165 (25 ng/mL). The vertical axis is the concentration of dextran. Empty bars, control conditions (5,5 or 25mM D-glucose); black bars, rVEGF165 (25 ng/mL); striped bars, Silymarin plus rVEGF165; gray bars, Silymarin. Results are expressed as mean±SD. *: p < 0.05.
Vascular leakage due to the breakdown of the BRB is the main event involved in the pathogenesis of DME 1,5. In the present study, we demonstrated that silymarin significantly prevented the VEGF-induced vascular hiperpermeability in HREC, which constitutes the inner BRB. It should be noted that the concentration of silymarin used was in the same range or even lower than reported in previous in vitro studies.21,25
In recent years three classes of drugs (renin-angiotensin [RAS] system blockers, fenofibrate and calcium dobesilate monohydrate [CaD]) have emerged as potential systemic treatments for DR. The antinflammatory and/or the antioxidant actions of these drugs have been reported among the underlying mechanism involved in their beneficial effects on DR. However, the current clinical evidence does not support the concept that RAS blockers possess an extra value in preventing or arresting the progression of DR in hypertensive patients when compared with other anti-hypertensive agents.26
Several sub-studies have demonstrated that fenofibrate (a PPARα agonist used as a hypolypemiant agent) has been useful in arresting the progression of DR but not in preventing its development and, therefore, it seems reasonable to propose its use for patients with preexisting DR.27 However, fenofibrate for DR treatment has been approved only in Australia, Singapore, Philippines and Malaysia, and a specific clinical trial aimed at determining its efficiency in preventing DR worsening is needed. Finally, CaD has been approved for the treatment of DR for many years in several countries but it has not been widely used for this purpose in clinical practice. A poor understanding of its mechanisms of action has been one of the reasons for this. However, recent experimental evidence unraveling multifaceted mechanism of action at retinal level seems to support clinical trials showing beneficial effects in early stages of DR.28
Silymarin is extracted from the plant Silybum marianum which is one of the most commonly plants used in liver diseases treatments. Silymarine is considered hepatoprotective and it has been widely used in patients with cirrhosis, chronic hepatitis and liver disease associated with alcohol consumption and exposure to environmental toxins.29 Currently, it is one of the most studied medicinal herbs for the treatment of non-alcoholic fatty liver disease (NAFLD) and steatohepatitis (NASH) and its use has been shown to be safe and well tolerated even for these patient groups. The therapeutic dose of silymarin described in clinical studies ranges between 200-800mg/day.30
We found that the highest concentration of silymarin evaluated in this study (10μg/ml) reduces HREC viability. In this regard, experimental evidence suggest that silymarin could be useful for cancer treatment in view of their pro-apoptotic effect, primarly p53 dependent 22, as well for its anti-angiogenic effects.23–25 It must be noted that these effects were observed in vitro in cultures of tumoral cells (colon, prostate, breast and skin cancer) using a concentration equal or higher than 40μg/ml. However, our results suggests that in HREC the threshold concentration to promote apoptosis is lower than in tumoral cells.
To the best of our knowledge, there are no studies on the anti-permeability effect of silymarin in endothelial cells. However, it has been reported that silymarin decreases the microalbuminuria in diabetic patients,18 which is closely related to endothelium impairment. We have found a strong inhibitory effect of slymarin on VEGF induced permeability, which is one of the most important pathogenic events in the development of DME. In addition, the antiangiogenic properties demonstrated in oncologic experimental studies point to silymarin as a potential candidate for treatment of proliferative diabetic retinopathy. Overall, these findings open up a new avenue in future research of this compound in the setting of either early and advanced stages of DR.
In conclusion, our results provided evidence that silymarin is effective to prevent BRB disruption, thus reducing vascular leackage. However, further studies to evaluate whether silymarin treatment could be useful for DME are needed.
Conflict of interestThe authors report no conflicts of interest related to this article
This study was supported by Global Remediation España S.A.