The use of continuous glucose monitoring (CGM) in type 1 diabetes (T1D) has been shown to reduce some acute complications, improve glycaemic control and improve patient satisfaction regarding its use.1–3 Recently, those countries that have introduced widespread public funding for these devices, such as the UK,4 Belgium,5 Sweden6 and France,7 have published their results available to date, confirming positive real-life outcomes in terms of HbA1c reduction, hospital admissions for acute complications and improved patient satisfaction compared with standard capillary blood glucose testing.
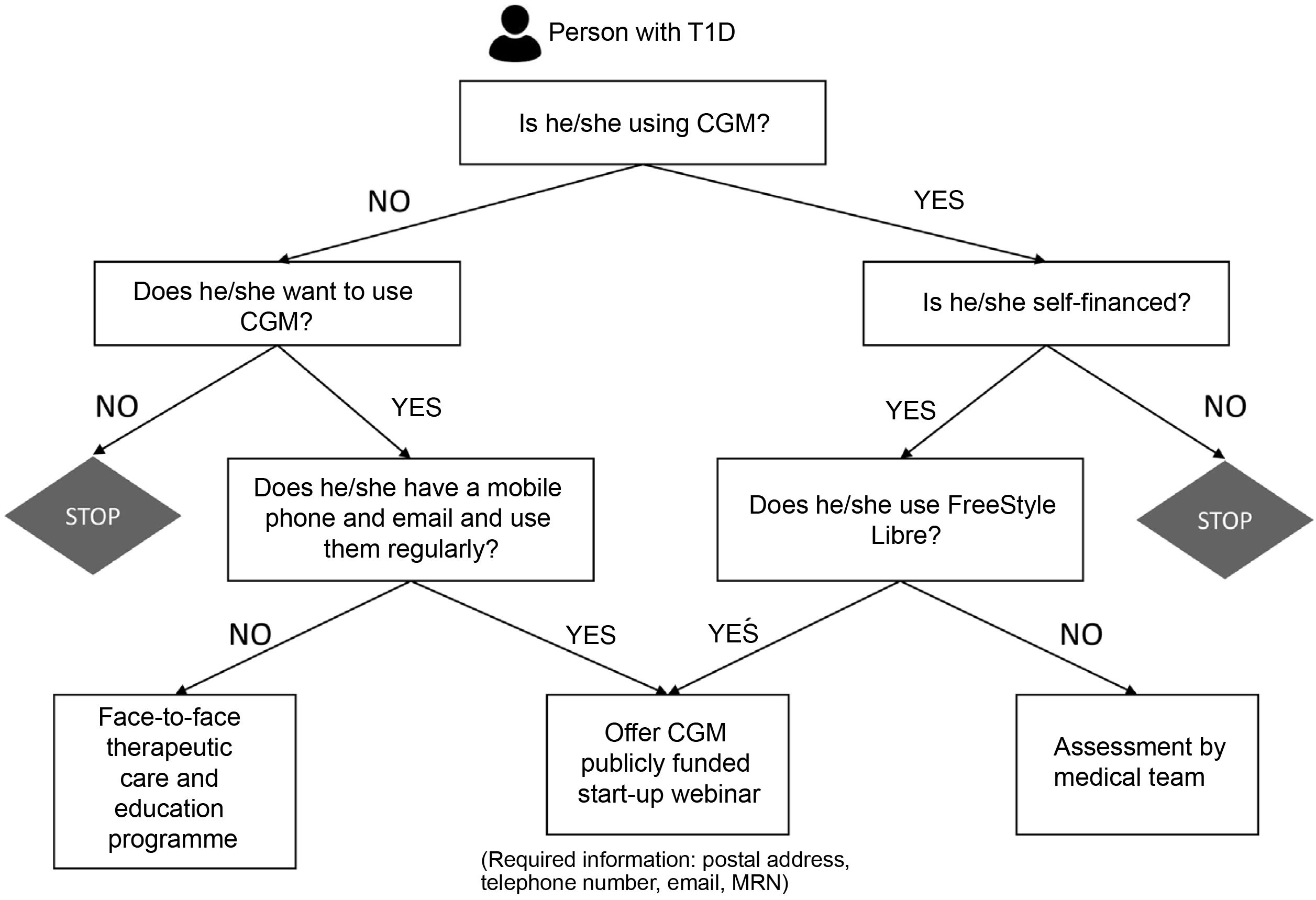
In Spain, public funding for CGM has been approached differently from one autonomous region to the next. In Catalonia, this funding was established in different phases, the latest of which included general funding for all patients with T1D.8 The large number of patients included in this last implementation phase led us to evaluate the feasibility and effectiveness of a decision algorithm (Fig. 1) aimed at the widespread implementation of CGM in people with T1D seen at our Diabetes Unit in the shortest possible time. In our case, the use of the FreeStyle Libre flash CGM device was prioritised.9 An administrative professional, supported by healthcare personnel, contacted the candidates and included them in the programme according to their technological skills. New users received device information, a contact phone number and a link to a training webinar. Those less familiar with technology and with the least technological skills received face-to-face training in small groups.
Over a period of three and a half months, from 1 March to 15 June 2021, 1,519 candidates were contacted by telephone (52% female, mean age 43.82±15.29 years, mean HbA1c 7.71%±1.19, 19% of whom were subcutaneous insulin pump users). A total of 1,045 patients (69%) started using the funded CGM, of whom 320 (21%) had previously self-financed the use of CGM; 331 people (22%) refused to use the device and 143 people (9%) could not be contacted. In webinars led by a diabetes nurse educator, 292 patients (29%) were included, while only 39 (3%) required face-to-face training. The majority of patients who refused to start CGM reported a lack of interest in using the device (45% of cases), while 17% preferred to make a decision after consulting their regular endocrinologist. No significant acute complications or relevant clinical issues were recorded. A modest increase in the number of device inquiries was observed (a total of 190 calls and 11 unscheduled face-to-face visits).
A Spanish study was recently published in this journal that showed that the incorporation of an educational programme in group and telematic format on the use of flash CGM devices, as part of the implementation strategies of these systems, is an effective option with associated benefits in terms of quality of life and fear of hypoglycaemia, which can be implemented in routine clinical practice in adult patients with T1D.10 Our study adds new information in this regard and demonstrates that the widespread implementation of funded CGM in the population with T1D in a short period of time is feasible, safe and effective through the use of coordinated strategies between healthcare and non-healthcare professionals, including face-to-face and virtual visits and online educational support.