The Prader-Willi syndrome (PWS) is a rare genetic disorder caused by absence of expression of the paternal alleles in region 15q11.2-q13. Obesity and hormonal deficiencies, especially of growth hormone (GH), are the most important signs from the therapeutic viewpoint. Recombinant GH (rGH) is effective in children and represents the mainstay in treatment; by contrast, little evidence in available in adult patients.
ObjectiveTo review the reported evidence on the beneficial and adverse effects of treatment with rGH in children and adults.
DesignA review was made of 62 original articles published between 2000 and 2017 using the PubMed database.
ResultsIn pediatric and adult PWS, rGH improves body morphology and composition, physical performance, cognition, psychomotor development, respiratory function, and quality of life with few adverse effects.
ConclusionsTreatment with rGH is effective and safe and improves quality of life in both children and adults with PWS.
El síndrome de Prader-Willi (SPW) es un trastorno genético raro causado por la ausencia de expresión de alelos paternos de la región 15q11.2-q13. La obesidad y los déficits hormonales, especialmente el de GH, son las manifestaciones con mayor implicación terapéutica. El tratamiento con GHr es efectivo en niños y supone un pilar en su tratamiento; no obstante, la evidencia en adultos es escasa.
ObjetivoRevisar la evidencia publicada en relación con los efectos beneficiosos y adversos del tratamiento con GHr en niños y adultos.
DiseñoRevisión bibliográfica de 62 artículos publicados entre los años 2000 y 2017, usando la base de datos PubMed.
ResultadosEn el SPW pediátrico y adulto la GHr mejora la morfología y composición corporal, el rendimiento físico, la cognición, el desarrollo psicomotor, la función respiratoria y la calidad de vida. Presenta pocos efectos adversos.
ConclusionesEl tratamiento con GHr es efectivo y seguro y mejora la calidad de vida en individuos con SPW.
The present review addresses the increasing interest in the rare disease known as Prader-Willi syndrome (PWS). Recombinant growth hormone (rGH) is proving to be a fundamental strategy in the multidisciplinary management of PWS in children, and there is growing evidence that it will also be so in adults. With the purpose of assessing the evidence regarding the beneficial and adverse effects of rGH in children and adults, we reviewed a total of 62 articles published between 2000 and 2017 and identified through a PubMed search.
OverviewPrader-Willi syndrome is a rare genetic disease1–3 caused by the absence of expression of paternal alleles in the region 15q11.2-q13.4 The incidence of PWS is 1/10,000–1/30,000 live births, with no differences in terms of gender or ethnic origin,1–4 and it is considered to be the most frequent cause of genetic obesity.5
The syndrome is characterized by a common clinical-behavioral phenotype, with slight differences depending on the causal genotype.1 The definitive diagnosis is currently established by DNA methylation testing, affording a sensitivity of 99%.4
The characteristic phenotypic alterations are secondary to hypothalamic dysfunction, causing hyperphagia, body temperature instability, high pain tolerance, hypersomnia and multiple endocrine alterations, including growth hormone (GH) and thyrotrophin deficiency, hypogonadism (50%) and stress central adrenal insufficiency.1,2,4,6,7
Growth hormone deficiencyThe GH deficiency (GHD) observed in PWS is independent of obesity and the causal genotype.8 It manifests as a decrease in spontaneous GH secretion, together with a quantitative and qualitative alteration of the response to secretory stimuli, whether natural (secretagogues) or artificial (stimulation tests). The GH response to stimulation is decreased in magnitude, less robust and delayed, and is associated with low plasma levels of insulin-like growth factor 1 (IGF-1).2,9
In children, GHD is observed in 40–100% of all cases of the disease1,2,9,10; more recent publications report prevalences of over 75%.11 The prevalence in adults has not been well established; some studies speak of 15–95%,10 while others report a prevalence of 8–38%.11 However, these results are derived from an altered response to a somatotropic axis stimulation test that is not always the same, since there is no gold standard in this respect. The most widely used test nowadays comprises stimulation with GHRH+arginine,9,10,12–16 though other tests of lesser sensitivity have also been used.9,12,17
Since the approval by the United States Food and Drug Administration (FDA) of rGH for the treatment of PWS-related growth failure in 2000, and by the European Medicines Agency (EMA) with the added indication of improved body composition in 2001,1–3,18 rGH has been used on a routine basis in the pediatric population. Children are considered to benefit from GH, both in the presence and absence of deficiency; stimulation tests for diagnosis and the start of therapy are therefore not necessary.3,9,11 By contrast, in adults the benefits of continuing treatment in the absence of deficiency are not so clear. Stimulation testing is, therefore, mandatory once end body height or adulthood has been reached, prior to GH therapy at adult doses being started.3,6,9,19
Growth hormone treatmentIn children, only genetic confirmation of PWS is required before replacement therapy can be started.2 Treatment proceeds gradually until the usual dose of 1mg/m2/day (≈0.035mg/kg/day)2,20–23 is reached. There is no evidence regarding the patient age indicated for starting therapy, though experts recommend the start of administration before hyperphagia and obesity develop (under 2 years).2,3,11,24 By contrast, treatment in adults should only be started with a diagnosis of GHD. The recommended starting dose is 0.1–0.2mg/day,10,11 with adjustment being advised according to the balance among the IGF-1 levels, the beneficial effects, and the presence of adverse effects. The IGF-1 levels should be maintained in the upper range of normal, between 0 and +2 standard deviations (standard deviation score [SDS]).1
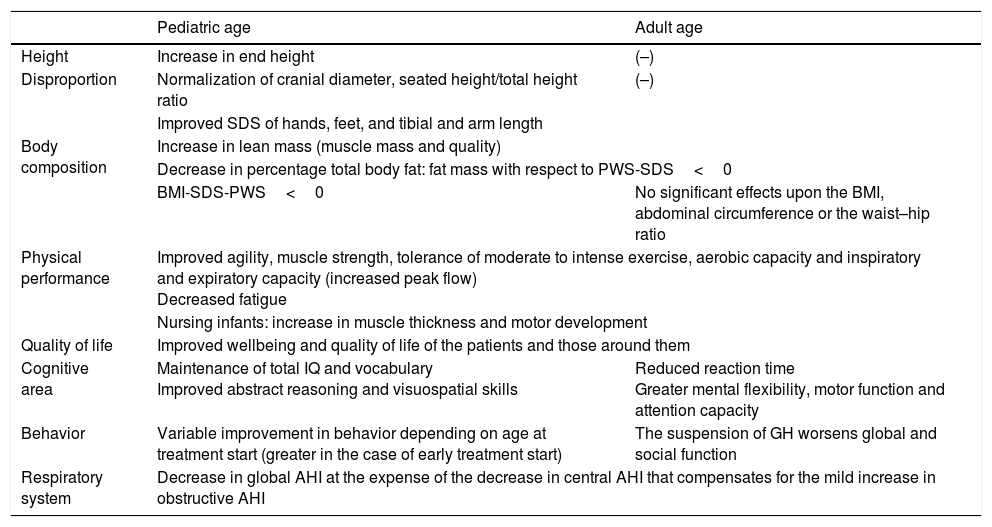
Benefits of recombinant growth hormone treatment in childrenThe effects of rGH therapy upon linear growth in children have been well established (Table 1). The introduction of rGH in infancy or in the prepubertal stage results in a linear increment with normalization of the growth rate and of end body height,6,8,20,21,24–26 reaching heights of 171±8cm in males and 158±4cm in females.27 When treatment is started later, in the pubertal stage, the increment is less consistent in terms of end body height.6 Some authors consider that although rGH normalizes end body height over the long term in relation to the normal population, the target height is not reached because of the absence of the pubertal growth spurt.26 Concomitant with the effect upon growth, patients experience complete normalization of the cranial circumference from one year of treatment26 and of the seated height/total height ratio,26 together with improved SDS of the hands, feet, and tibial and arm length, though without normalization at four years.26
Beneficial effects of growth hormone treatment in children and adults.
| Pediatric age | Adult age | |
|---|---|---|
| Height | Increase in end height | (–) |
| Disproportion | Normalization of cranial diameter, seated height/total height ratio | (–) |
| Improved SDS of hands, feet, and tibial and arm length | ||
| Body composition | Increase in lean mass (muscle mass and quality) | |
| Decrease in percentage total body fat: fat mass with respect to PWS-SDS<0 | ||
| BMI-SDS-PWS<0 | No significant effects upon the BMI, abdominal circumference or the waist–hip ratio | |
| Physical performance | Improved agility, muscle strength, tolerance of moderate to intense exercise, aerobic capacity and inspiratory and expiratory capacity (increased peak flow) Decreased fatigue | |
| Nursing infants: increase in muscle thickness and motor development | ||
| Quality of life | Improved wellbeing and quality of life of the patients and those around them | |
| Cognitive area | Maintenance of total IQ and vocabulary Improved abstract reasoning and visuospatial skills | Reduced reaction time Greater mental flexibility, motor function and attention capacity |
| Behavior | Variable improvement in behavior depending on age at treatment start (greater in the case of early treatment start) | The suspension of GH worsens global and social function |
| Respiratory system | Decrease in global AHI at the expense of the decrease in central AHI that compensates for the mild increase in obstructive AHI | |
IQ: intelligence quotient; AHI: apnea–hypopnea index; SDS: Standard Deviation score; PWS: Prader-Willi syndrome.
Although the changes in body composition are already apparent in nursing infants,4 there is little evidence of the effects of treatment at that age. Bakker et al.20 carried out a clinical trial in this population and found rGH to maintain percentage total fat after one year of treatment, while the corresponding percentage in the control group was seen to increase.24 On the other hand, Eiholzer25 reported that the early introduction of rGH could increase lean mass23,25 to normal levels and delay the accumulation of fat mass. Starting from the prepubertal stage, treatment over the short term increases lean mass and lowers the percentage total fat mass and the body mass index (BMI).20,21,26,28,29 After four years of treatment the positive effects upon body composition are maintained, though after 8 years a loss of effect is noted, with stabilization of the lean mass23 at levels above the basal levels (without reaching normalization) and a progressive worsening of the percentage total fat mass to levels comparable to those at the start of treatment. Although to a less intense extent, the effect upon patient BMI is seen to persist; after 8 years this parameter is significantly lower than in the untreated PWS controls.23
Body composition responds early to treatment with rGH, and this response persists for the duration of therapy at full doses.25,26 Adults treated with rGH in childhood show improved body composition (lesser weight, BMI and prevalence of obesity) and metabolic status (lower HbA1c, HOMA-IR and arterial hypertension) compared with those who were not treated.30 Sudden treatment suspension results in a rapid worsening of these parameters, however.21
Recombinant growth hormone has no known effect upon bone mineral density (BMD), which proves normal until puberty when adjusted for height. The situation subsequently worsens, however, presumably in relation to hypogonadism.7,26,31
By contrast, rGH has a positive effect upon physical performance: treatment improves agility, muscle strength and coordination, reduces fatigue and increases tolerance of exercise, thereby improving hypokinetic syndrome and the inspiratory and expiratory capacity of the patient.21,23,28,29
In terms of cognitive function, rGH exerts positive effects upon some areas of psychomotor development.23,26,32 Over the short term, treatment prevents the deterioration of vocabulary and abstract verbal reasoning. Over the long term, and in addition to maintaining the total intelligence quotient (IQ) and vocabulary, it improves abstract verbal reasoning, visuospatial skills and total IQ.27,32 The lower the initial score and the earlier the start of therapy, the greater is the observed increment. In fact, starting therapy in prepubertal children stabilizes the IQ instead of improving it.27,32 In parallel to the above, rGH therapy improves the quality of life of the patients and those around them.21
The effect of rGH upon the respiratory disorders of PWS is a subject of some controversy. In the period between 2002 and 2005, multiple cases of sudden death were reported associated with the start of treatment with rGH that were attributed to respiratory failure.18,33,34 Subsequently, in 2008, a review was published documenting 64 sudden deaths, of which 61% were attributable to respiratory causes (respiratory failure or infections). Since then no further studies on this subject have been carried out. Although these studies were unable to demonstrate a causal relationship, different clinical guides,3 reviews1,4–9,11 and original articles18 have made recommendations based on these data. However, studies specifically focused on sleep apnoea–hypopnoea syndrome (SAHS) have demonstrated an improvement in the global apnea–hypopnea index (AHI) at the expense of an increased reduction of the central apneic episodes that compensate for the worsening of obstructive AHI.18,35–38
There is little evidence regarding the alteration of cardiac function in PWS. In any case, such alterations are very mild, of a subclinical nature, and are only observed on the electrocardiogram and by echocardiographic exploration. To date, one retrospective study39 in the pediatric population has been published, documenting a tendency toward echocardiographic improvement of diastolic function following treatment with GH.
Adverse effects of recombinant growth hormone treatment in childrenReplacement therapy with rGH is generally well tolerated. The adverse effects are few, mainly mild, and usually do not require treatment discontinuation (Table 2).18,26,40
Adverse effects of growth hormone treatment in children and adults.
| Pediatric age | Adult age | |
|---|---|---|
| Adenoid hypertrophy/SAHS | No correlation with IGF-1 levels | Improvement of SAHS (scant evidence) |
| Recommendation of polysomnography, ENT evaluation and treatment of obstructive SAHS before therapy with GH is started | ||
| Sudden death | No increase in global mortality, though concentrated in the first 9 months of treatment | (–) |
| Hyper-IGF1 | No clinical effects | GH dose according to IGF-1 and clinical effects |
| Metabolic homeostasis | First two years of treatment: slight increase in insulinemia, blood glucose, HOMA-IR; no increase in HbA1c, the incidence of DM2 or OGI in patients who comply with the hygiene-dietetic indications No increase in total cholesterol, HDLc or LDLc No increase in blood pressure | |
| (–) | No increase in the incidence of metabolic syndrome | |
| Others | (–) | Increase in total body water, edemas Headache Muscle and joint pain |
DM2: type 2 diabetes mellitus; OGI: oral glucose intolerance; ENT: (ear, nose and throat): SAHS: sleep apnea–hypopnea syndrome.
The effect generating most concern within the scientific community is the increase in serum IGF-1 to supratherapeutic levels. A number of publications have described a specific hypersensitivity of PWS to treatment, generating a disproportionate increase in IGF-1,18,22,26 which can be corrected by lowering the rGH dose.26 However, rGH doses of under 1mg/m2/day are unable to produce optimum effects, thereby resulting in a worsening of body composition.26 It should be noted that even with IGF-1 levels above normal, no acromegaloid growth disturbances22 or direct correlation with tonsillar and adenoid hypertrophy have been observed.18 Some authors postulate that this is because serum IGF-1 concentration is not a good indicator of bioactivity, and recommend the IGF-1/IGFBP3 ratio for therapeutic monitoring purposes.22
Berini et al.18 analyzed the effect of rGH upon respiratory function during sleep, and although they detected a significant relationship between the development of obstructive SAHS and adenoid size, no such correlation with IGF-1 concentration was observed. On the other hand, Meyer et al.41 found that although tonsil and adenoid removal improved mild to moderate obstructive SAHS, it had no impact upon severe presentations of the disorder. The authors postulated that in these latter cases adenoid hypertrophy (and therefore GH treatment) is not the cause. Nevertheless, a safety alert persists regarding the possible respiratory effects, due to the review mentioned above, citing cases of sudden death (61%),35 and to the fact that the incidence is concentrated mainly in the first 9 months of treatment.33,42 For this reason, experts3,18 recommend a close monitoring of respiratory function before treatment is started and also one year later, based on ear, nose and throat evaluation and a polysomnographic study. In the event that obstructive SAHS is detected, it should be treated (topic corticosteroids, tonsil and adenoid removal and continuous positive airway pressure [CPAP] in refractory cases) before rGH treatment is started.18,41
With regard to metabolic homeostasis, rGH does not appear to affect blood glucose or lipid metabolism. Although a slight increase is observed in blood glucose, insulinemia and insulin resistance (HOMA-IR) from the prepubertal stage,24 these changes are not reflected in the HbA1c concentration.18,26,40 There is no evidence that rGH results in earlier onset diabetes or an increased risk of suffering diabetes43; in fact, the incidence of oral glucose intolerance is lower in individuals receiving or who have received rGH than in those who have never received such treatment.40 Likewise, rGH has not been found to worsen hyperphagia20 or other safety parameters such as blood pressure, total cholesterol or LDLc and HDLc levels. Indeed, some studies have even reported improvements in the patient lipid profile.24,26
For a long time scoliosis was regarded as a contraindication to GH therapy. However, it has been seen that such therapy does not influence the prevalence, onset or progression of scoliosis.24,26,44
Lastly, although a possible increase in cancer risk has been reported in patients with antecedents of cancer who received rGH in childhood,45 there is insufficient evidence to warrant disadvising the use of rGH in such patients.
Benefits of recombinant growth hormone treatment in adultsThe effects of treatment in adults have been less characterized than in the pediatric population, particularly among those over 40 years of age. Nevertheless, evidence has been obtained in recent years suggesting the effectiveness and safety of treatment during this period of life (Table 1).
Globally, the benefits observed in adulthood are similar to those seen in the pediatric population. Recombinant growth hormone increases lean mass, improves muscle mass and quality, and reduces percentage total fat, subcutaneous fat and abdominal visceral adiposity.12–14,17 Treatment does not significantly modify the BMI, abdominal circumference, the waist–hip ratio or basal metabolism.12,13 Anabolic effects are observed from the first year of treatment, persist throughout therapy,12,17 and are seen in both patients with and without GH deficiency.46–48 Treatment suspension results in worsened body composition, with an increase in the BMI at the expense of body fat, particularly at the visceral level.13
Altered bone metabolism is exclusive to the adult stage in PWS. While BMD is normal in the prepubertal stage and is not modified by rGH therapy,7,26,31 a gradual decrease in BMD is seen during puberty, leading to the development of osteoporosis and an increased risk of fractures in adult life. In this case, although rGH does not increase BMD,12,15,26,31 it does ameliorate bone geometry.15
In parallel to the above, rGH therapy improves the quality of life of the patients and those around them. Treatment improves tolerance of moderate to intense exercise, aerobic capacity, muscle strength and energy,12,14,46,47 which in turn has a long-term impact upon lung function, with an increase in peak expiratory flow.10,12,47
As commented above, PWS is characterized by a series of very subtle functional and structural cardiac alterations similar to those found in other types of diseases involving GH deficiency. Recombinant GH therapy in adults increases left ventricular mass without significant changes in systolic or diastolic function.14,49 While this is statistically so, both functions tend to decrease14; echocardiographic monitoring is therefore advised in long-term treatments.
In parallel to the above, rGH improves mental agility and flexibility, reaction time, motor function, and sustained attention capacity during treatment,12,32,48,50 and moreover increases patient wellbeing and quality of life.12,32,50 The suspension of rGH leads to a rapid worsening of physical and social function and global performance.12
Adverse effects of recombinant growth hormone treatment in adultsIn view of the inherent insulin countering effect of rGH and the tendency toward the progression of obesity in PWS, with the consequent intrinsic increase in peripheral insulin resistance, the repercussions of rGH therapy upon glucose metabolism have been widely studied in the adult population (Table 2).12–14,16,17,26,40,51 Most short-term studies describe a slight increase in blood glucose, basal insulinemia and HOMA-IR, independently of the BMI,16 with values that remain within normal limits and have no effects upon HbA1c.12–14,16,17,52 By contrast, in one study a minimum but significant increase (0.2%) in HbA1c was found after two years of treatment. Likewise, some patients developed oral glucose intolerance13 and type 2 diabetes mellitus (DM2) at the start of treatment. However, these latter individuals presented oral glucose intolerance at baseline52 and experienced a considerable increase in body weight as a result of non-compliance with the dietetic and physical exercise instructions during therapy.53 Recombinant GH therefore should not be administered to patients that are unlikely to comply with the concomitant hygiene-diet recommendations.53 Blood glucose control in patients with background DM2 proved more difficult in some individuals,13 though levels within normal limits were maintained over the long term.53 In contrast to the short-term situation, long-term therapy has no adverse effects upon glucose metabolism. Insulin resistance (HOMA-IR) tends to increase with age26,40; in this regard, on adjusting blood glucose, insulinemia, HOMA-IR and HbA1c to patient age, the values do not correlate with rGH therapy or the IGF-1 levels.26 In fact, a study found that patients who had been treated with rGH presented a lesser prevalence of oral glucose intolerance than those who had never received such treatment.40 The lipid profile and blood pressure values likewise experienced no significant alterations as a result of treatment with rGH,12,14,26 in contrast to metabolic syndrome (MS). In this case the evidence is slightly contradictory: while one study54 reported a decrease in the incidence of MS, another17 reported an increase in triglycerides and body weight associated with dietetic non-compliance, thereby implying that MS is not related to treatment with rGH.
With regard to sleep respiratory disturbances, very little information regarding treatment with rGH is available in adults. In fact, some studies which focus on the adult population use pediatric references to establish their recommendations.9,28,29 All the existing evidence comes from the short-term study carried out by Miller et al.36 involving 10 adults with PWS. The authors performed polysomnography at the start of treatment with rGH and again after 6 weeks. Only one of the 10 patients showed a worsened obstructive apnea–hypopnea index (AHI), and the overall group showed a decrease in global AHI, as well as a tendency toward improved central AHI. In view of the scant intrinsic evidence available, the pediatric recommendations are still extrapolated from this population, implying prior respiratory evaluation and treatment of the cases of severe SAHS with CPAP (elective in adults) before therapy with rGH is started.1,3
Together with blood glucose homeostatic alterations, the appearance or worsening of edemas of the lower extremities is the most frequently reported adverse effect. These edemas appear during the first month of treatment,14 are generally mild,13 located in the ankles and pretibial region,13,17 and generally require no dose adjustment or treatment suspension.12,13 They are associated with a tendency toward increased total body water content,12,14,17 and only caused treatment discontinuation in one patient.17
On an anecdotal basis, there have been reports of muscle pain,12,17 joint pain14 and headache with nausea12,13 in adults with PWS subjected to rGH therapy.
Finally, an increased risk of myeloid leukemia has been described in adults with PWS who have not received rGH, and which could be associated with the genetic defect in chromosome 15q. In fact, alterations in chromosomal region 15q have been reported in myeloid and lymphoblastic leukemia.55 However, prospective studies are needed in order to better calibrate the true risk of leukemia in these patients.
ConclusionsTreatment with rGH starting at an early patient age favorably modifies the natural course of PWS. Although the evidence in adults is scarce, rGH therapy has been seen to be effective and safe, and improves the quality of life both of the patients (both adults and children) and of those around them. There are no scientific evidence-based contraindications restricting the use of rGH in PWS, and no clear evidence relating rGH to increased mortality.
Since most of the existing studies are not controlled and are of short duration, long-term controlled trials are recommended, particularly in adults, in order that firm conclusions can be drawn as to the safety and effectiveness of this treatment.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Moix Gil E, Giménez-Palop O, Caixàs A. Tratamiento con hormona de crecimiento en el síndrome de Prader-Willi. Endocrinol Nutr. 2018;65:229–236.