Prader–Willi Syndrome (PWS) is the most common genetic cause of obesity, occurring in approximately 1 in 15,000 newborns. It results from the lack of expression of genes on the paternal allele of the chromosomal region 15q-11q13 (65–75% due to type 1 or type 2 deletion). Individuals with PWS experience associated symptoms such as hypotonia, hyperphagia, and early-onset obesity (before 5 years of age). Around 20% of adults with PWS also develop type 2 diabetes. Previous studies have shown the beneficial effects of GLP1-RA medications, such as exenatide and liraglutide, in treating type 2 diabetes in PWS. However, there is limited information available on the use of semaglutide in PWS. This study aimed to evaluate the effects of semaglutide on weight loss and glycaemic control in four patients with PWS and type 2 diabetes associated with obesity. The patients were started on weekly subcutaneous progressive doses of semaglutide.
El síndrome de Prader-Willi (SPW) es la causa más común de obesidad genética, con una incidencia de 1:15.000 recién nacidos. Se produce por una falta de expresión de los genes ubicados en el alelo paterno de la región cromosómica 15q-11q13 (65-75%) debido a la deleción tipo 1 o tipo 2. Presentan hipotonía, hiperfagia y obesidad de inicio temprano (antes de los 5 años de edad). Hasta el 20% de los pacientes en la edad adulta tienen diabetes tipo 2. Se han descrito efectos beneficiosos de GLP1-RA, exenatida y liraglutida en el tratamiento de la diabetes tipo 2 en este síndrome.
Actualmente hay poca información sobre el uso de semaglutida en el SPW. El objetivo fue evaluar el efecto de semaglutida en la pérdida de peso y el control glucémico en una serie de 4 pacientes con SPW y diabetes tipo 2 asociada a obesidad que comenzaron con semaglutida subcutánea semanal en dosis progresivas.
Prader–Willi Syndrome (PWS) is the most prevalent cause of syndromic obesity, estimated to affect 1 in 10,000 to 1 in 30,000 newborns. It arises due to the absence of gene expression from the paternal origin within the 15q11-q13 chromosomal region. Approximately 65–75% of cases result from a deletion (type I and type II), while maternal uniparental disomy accounts for 20–30%, and an imprinting defect account for 1–3%.1 PWS is characterized by persistent neonatal hypotonia, short stature due to growth hormone deficiency, hypogonadism, varying degrees of intellectual disability, and hyperphagia starting in early childhood (before the age of 5), leading to severe obesity.2–4 Obesity is the primary cause of increased morbidity and mortality in individuals with this syndrome. Diabetes typically manifests in 10–25% of PWS patients, typically during adulthood. Paradoxically, despite severe obesity, relative hypoinsulinemia is frequently observed.5
The exact pathophysiology of obesity in PWS is not fully understood but is likely the result of a combination of hypothalamic dysfunction leading to hyperphagia, dysregulation of hormones controlling hunger and satiety, reduced baseline energy expenditure, and deficiencies in various hormones (e.g., growth hormone, hypogonadism, hypothyroidism).
Obesity affects approximately 40% of children and adolescents with PWS and increases to over 90% in adulthood.6–8
Previous studies have shown the beneficial effects of an analogue of glucagon-like peptide-1 (GLP1-RA) medications, such as exenatide and liraglutide, in treating type 2 diabetes in PWS. However, there is limited information available on the use of semaglutide in PWS. This study aimed to evaluate the effects of semaglutide on weight loss and glycaemic control in four patients with PWS and type 2 diabetes associated with obesity.
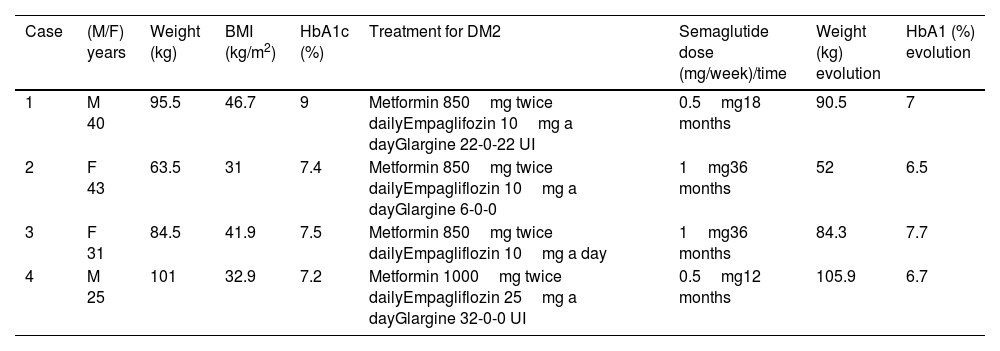
Description of cases and managementTable 1 summarizes the characteristics of the patients.
Characteristics of patients.
| Case | (M/F) years | Weight (kg) | BMI (kg/m2) | HbA1c (%) | Treatment for DM2 | Semaglutide dose (mg/week)/time | Weight (kg) evolution | HbA1 (%) evolution |
|---|---|---|---|---|---|---|---|---|
| 1 | M 40 | 95.5 | 46.7 | 9 | Metformin 850mg twice dailyEmpaglifozin 10mg a dayGlargine 22-0-22 UI | 0.5mg18 months | 90.5 | 7 |
| 2 | F 43 | 63.5 | 31 | 7.4 | Metformin 850mg twice dailyEmpagliflozin 10mg a dayGlargine 6-0-0 | 1mg36 months | 52 | 6.5 |
| 3 | F 31 | 84.5 | 41.9 | 7.5 | Metformin 850mg twice dailyEmpagliflozin 10mg a day | 1mg36 months | 84.3 | 7.7 |
| 4 | M 25 | 101 | 32.9 | 7.2 | Metformin 1000mg twice dailyEmpagliflozin 25mg a dayGlargine 32-0-0 UI | 0.5mg12 months | 105.9 | 6.7 |
Abbreviations: BMI, body mass index; HbA1c, glycosylated haemoglobin; DM2, type 2 diabetes mellitus.
Written informed consent was obtained from all patients and/or parents or legal representatives.
Case 1: A 40-year-old man with PWS, resulting from a type II deletion, visited our Specialized Unit. His medical history included hypogonadism, asthma, obstructive sleep apnoea syndrome (treated with CPAP), and scoliosis. He initiated growth hormone (GH) five years ago (0.8mg per week), having not received GH during childhood. He developed type 2 diabetes at the age of 35 without any chronic complications. His diabetes treatment consisted of glargine insulin (22 units twice daily), empagliflozin (10mg daily), and metformin (850mg twice daily). At the beginning of the study, his weight was 95.5kg, height 143cm, BMI 46.7kg/m2, and glycated haemoglobin was 9%. The Endocrinologist added subcutaneous semaglutide 0.25mg per week to his treatment, which was later increased to 0.5mg per week after a month. After six months of treatment with semaglutide 0.5mg per week, he achieved a weight loss of 5kg and improved glycaemic control with a glycated haemoglobin of 7%. This improvement was maintained one year later. Subsequently, he was advised to increase the dose to 1mg, but he was unable to start due to lack of supply. As a result, he switched to liraglutide at a dose of 1.8mg daily, which led to weight gain and worsening of glycaemic control.
Case 2: A 43-year-old woman with PWS, resulting from a type I deletion, visited our Specialized Unit. Her medical history included hypogonadism, obstructive sleep apnoea syndrome (treated with CPAP), scoliosis, and bacterial endocarditis requiring surgical intervention. Similar to Case 1, she did not receive GH treatment during childhood but started GH at adult doses five years ago (1.4mg per week). She developed type 2 diabetes at the age of 33 without any chronic complications, and her glycated haemoglobin was relatively well controlled at 7.4%. At the start of the study, she was being treated with metformin (850mg twice daily), empagliflozin (10mg daily), and glargine insulin (6 units at bedtime). Her weight was 63.5kg, height 143cm, and BMI 31kg/m2. Semaglutide 0.25mg per week was added to her treatment, this dose was well tolerated, so she increased the dose to 0.5mg per week and finally to 1mg per week. She improved glycaemic control, stopped insulin and achieved a glycated haemoglobin of 6.5% and lost weight up to 48.5kg after 6 months with the last dose change, 24 months after the beginning of semaglutide. However, she regained weight up to 52kg at 36 months with the same glycated haemoglobin.
Case 3: A 31-year-old woman with PWS, resulting from a type I deletion, visited our Specialized Unit. She did not receive GH treatment during childhood but started GH at adult doses 5 years ago (2.8mg per week). She had type 2 diabetes with suboptimal control (glycated haemoglobin of 7.5%) under treatment with metformin 850mg twice daily and empagliflozin 10mg a day. Before starting with semaglutide, her weight was 84.5kg (BMI: 41.9kg/m2). The initial dose of semaglutide was 0.25mg subcutaneously per week and one month later increased to 0.5mg per week. After 12 months she lost 2kg and her glycated haemoglobin was 6.9%. Later on, she increased her weight 2kg so the physician increased semaglutide dose to 1mg per week but maintained the same weight and the glycaemic control deteriorated reaching again a glycated haemoglobin of 7.7%.
Case 4: A 25 years old man with PWS due to a deletion type II was visited in our Specialized Unit. He had not received GH treatment during childhood. He started GH treatment 3 years ago at adult doses 1.4mg per week. He had type 2 diabetes and obesity (weight 101kg, height 175, BMI: 32.97kg/m2). His treatment was: glargine insulin 32UI before breakfast, metformin 1000mg twice daily and empagliflozin 25mg a day. His glycated haemoglobin was 7.2%. After 6 months with semaglutide 0.25mg a day, his weight and glycaemic control did not change. His physician encouraged him to increase semaglutide dose but he refused and stopped the treatment. Later on, he resumed treatment with semaglutide and increased the dose to 0.5mg per week. After 6 months with this dose, his glycated haemoglobin dropped from 7.4% to 6.7% and his weight increased from 1015kg to 105.9kg.
DiscussionIn this investigation, we assessed the impacts of semaglutide, an analogue of glucagon-like peptide-1 (GLP1- RA), on the reduction of weight and regulation of blood sugar levels in four adult patients diagnosed with Prader–Willi Syndrome (PWS) and type 2 diabetes. In all patients, semaglutide once a week was well tolerated. The first two patients (cases 1 and 2) experimented an improvement of weight and glycaemic control. Case 3 experimented an initial improvement in weight and glycaemic control but with later regain of weight and a deterioration of glycated haemoglobin. Finally, case 4 experimented an improvement in glycaemic control but with weight gain.
Numerous clinical trials have been conducted to date, using different medications aiming to reduce hunger in these patients, but without success.9
GLP1-RA are increasingly being employed to treat obesity in patients with or without type 2 diabetes. Glucagon-like peptide is a gut hormone produced by L-cells in response to food intake.10 Its functions include stimulating insulin secretion, inhibiting glucagon release, delaying gastric emptying, and promoting satiety after meals. Impaired secretion and action of GLP-1 have been implicated in the pathophysiology of obesity and type 2 diabetes. Exenatide, approved by the US Food and Drug Administration (FDA) in 2005, was the first aGLP-1 medication used to treat type 2 diabetes.11 More recently, liraglutide has been approved by the FDA and the European Medicines Agency (EMA) for obesity treatment (without diabetes) in adults and adolescents.12,13
GLP1-RA has been used with some success in patients with hypothalamic obesity,14,15 making it a potentially viable option for individuals with PWS. Liraglutide specifically acts on the pro-opiomelanocortin and cocaine-amphetamine-regulated transcript (POMC/CART) neurons within the arcuate nucleus, the primary centre regulating appetite in the hypothalamus.16 Furthermore, studies have shown that liraglutide reduces levels of ghrelin, an appetite-stimulating peptide that is elevated in PWS during fasting and after meals.17
The results of the main studies carried out in patients with PWS with GLP1-RA are summarized below. Some explored the effect on weight and appetite in obese PWS patients without diabetes mellitus18,19 and others also explored the effect on glycaemic control.20,21
Diene et al.18 studied the effect of GLP1-RA on weight with liraglutide treatment compared with placebo in paediatric individuals (31 adolescents and 24 children) with PWS and obesity in a in a multicentre, 52-week, placebo-controlled trial with poor results. The change in BMI standard deviation score from baseline to weeks 16 and 52 did not differ significantly between the treatment and placebo groups in both adolescents (estimated treatment difference: −0.07 at week 16 and −0.14 at week 52) and children (−0.06 and −0.07, respectively). However, at week 52, treated adolescents exhibited lower hyperphagia total and drive scores compared to the no-treatment group. The most common adverse events associated with liraglutide treatment were gastrointestinal disorders.
Goldman et al.19 reported a series of cases and conducted a literature review on the use of anti-obesity medications (AOMs) in children and adolescents with PWS and obesity. The case series included PWS patients with obesity but without diabetes who were treated with metformin, topiramate, liraglutide, and semaglutide. The cases demonstrated that patients with PWS and obesity can benefit from various AOMs with minimal adverse effects and varying degrees of weight reduction. Two 12-year-old males in the series were treated with subcutaneous semaglutide 1mg per week for 16 weeks as part of combination therapy (including metformin+topiramate). One patient experienced an increase in zBMI (+0.02) and weight (+2.4kg) at the fourth month compared to baseline, while the other patient showed a decrease in zBMI (−0.02) and weight (−5.7kg) during the same period.
The literature review conducted by Goldman et al.19 included studies involving PWS patients with obesity (some with diabetes) who were treated with topiramate, metformin, phentermine, orlistat, liraglutide, oxytocin, naltrexone-bupropion, or various combinations of these medications. The review revealed improvements in hyperphagia and social behaviour, but the effects on BMI or weight loss were variable and limited. Liraglutide, often used in combination with other anti-obesity medications like metformin or empagliflozin, demonstrated the most promising effects on weight loss. The studies on liraglutide often reported decreases in HbA1c (ranging from −1.3% to −1.9% over 14–16 weeks or no decrease) and variable effects on weight (ranging from −3.2kg to no change) without significant side effects.
Beng et al. conducted a systematic review examining the impact of aGLP1 (exenatide and liraglutide) on weight loss and glycaemic control in Prader–Willi Syndrome.20 The review included ten studies, involving a total of 23 PWS patients (aged 13–37 years) who were treated with either exenatide (n=14) or liraglutide (n=9) for durations ranging from 14 weeks to 4 years. Of these patients, 16 (70%) had type 2 diabetes. Ten patients experienced improvements in body mass index (BMI), ranging from 1.5 to 16.0kg/m2, while 19 of the 23 cases showed improvements in HbA1c levels, ranging from 0.3% to 7.5%. Appetite and satiety levels were reported to improve in all five studies. No serious side effects were reported.
Finally, Sani et al.21 reported a case of a 33-year-old man with Prader–Willi Syndrome complicated by poorly controlled diabetes and severe obesity. This individual was treated with subcutaneous semaglutide 1mg per week. After 12 months of treatment, significant reductions in glycated haemoglobin levels (from 11.1% to 7.2%) and body weight (from 99.5kg to 94.3kg) were observed, along with a notable decrease in fat mass and insulin requirements. It is worth noting that this patient had previously tried liraglutide therapy in combination with metformin and insulin without significant efficacy.
As in our series, the results in terms of weight loss and improvement in glycaemic control were heterogeneous in those patients treated with GLP1-AR. This circumstance can also be observed in routine clinical practice in patients with obesity and type 2 diabetes mellitus without PWS in terms of glycaemic control. Current evidence on treatment effect heterogeneity for this therapy is limited, possibly reflecting the methodological limitations of published studies so far.22
In summary, the use of GLP1-RA medications, such as liraglutide and semaglutide, has shown some promising effects in the treatment of obesity and glycaemic control in individuals with PWS. These medications have demonstrated improvements in BMI, HbA1c levels, and satiety levels in some individuals. However, it is important to note that the long-term effects and efficacy of GLP1-RA in PWS are still uncertain and robust and appropriately powered studies are required to understand treatment effect heterogeneity and evaluate the potential for precision medicine to provide future clinical care.
Areas of uncertaintyTaking into account this series of cases and the literature review, it can be deduced that managing diabetes and obesity in individuals with Prader–Willi Syndrome (PWS) is challenging due to the inherent food behaviour associated with hyperphagia. The long-term effects of GLP1-RA in PWS remain uncertain, and further studies are needed.
ConclusionThe treatment of hyperphagia in PWS remains a challenge. Semaglutide, administered once weekly, can be effective and safe for some patients with PWS and type 2 diabetes mellitus associated with obesity.
Guidelines and recommendationsWhile there is no specific treatment for diabetes and obesity in PWS, we encourage the use of medications that have a favourable effect on weight loss, despite the possibility of short-lived effects.
Declaration of generative AI and AI-assisted technologies in the writing processDuring the preparation of this work the author(s) used ChatGPT in order to Improve language. After using this tool/service, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the publication.
FundingNo grants supported the writing of this manuscript.
Conflict of interestThe authors declare no conflicts of interest.
We thank the patients and their families.