Hemoglobin A1c (HbA1c) levels have been linked to the development and progression of chronic complications in diabetes mellitus. Consequently, lowering HbA1c levels is the main treatment goal in diabetic patients, and determination of this hemoglobin (Hb) is the method regularly used to monitor glucose control in these patients.
HbA1c may be falsely low in certain clinical situations, some of which are quite obvious (e.g., pregnancy, recent bleeding, hemolytic anemia, iron replacement therapy or treatment with vitamin B12).1 However, HbA1c levels can also be altered in silent conditions which, if undetected, can lead to improper management of the diabetic patient. Many of these conditions are related to hemoglobinopathies.2
We describe a case of abnormally low HbA1c that occurred in a family without any associated clinical manifestations, in which hemoglobin (Hb) J-Baltimore was identified. The index case was a 35-year-old Caucasian woman with no relevant personal or family history, who presented with typical diabetes mellitus symptoms. She was referred to our hospital, showing glucose 426mg/dl and HbA1c 10.9%(by turbidimetric inhibition immunoassay [TINIA]; normal value [NV]: 4.5–6%). The findings of physical examination were normal.
After 3 months of oral treatment, a new biochemical analysis showed glucose 152mg/dl, HbA1c 4.1% (by high-performance liquid chromatography [HPLC]; NV: 4.5–5.5%), HbF 2.4% (NV: <0.5%) and a normal complete blood count. The patient denied having had any hypoglycemic episodes and self-monitoring of capillary blood glucose showed levels greater than 130mg/dl at all times. Pregnancy was ruled out, and she presented no data suggesting other associated disorders.
A cytogenetic and hematological study was performed on the patient and her family. HbA1c was determined at our hospital by HPLC (Adams HA-8160, Menarini). Previously, HbA1c had been measured by TINIA (Hitachi 917, Roche).
The hemoglobin study consisted of an electrophoresis on cellulose acetate at alkaline pH (pH 8.6), isoelectric focusing in polyacrylamide gel (pH 5.5–8.5), citrate-agar electrophoresis (pH 6), reverse-phase HPLC of globin chains, and cation-exchange HPLC. Hemoglobin stability was verified by means of the isopropanol test, and its function by determining P50 on the oxygen equilibrium curve using a TCS Hemox Analyzer (TCS Medical Products Co., Huntingdon Valley, PA, USA).
The molecular analysis was performed by sequencing the PCR amplification products of the β globin gene by means of automatic sequencing using the ABI Prism™ dRhodamine Terminator Cycle Sequencing Ready Reaction Kit (PE Applied Biosystems, Foster City, CA). The reaction sequence was analyzed using an ABI Prism 310 Genetic Analyzer.
The family study revealed abnormally low HbA1c levels by HPLC in the mother and daughter of the index case. The father's HbA1c levels were normal, and his study was stopped.
The results of the index case, mother and daughter were as follows:
- •
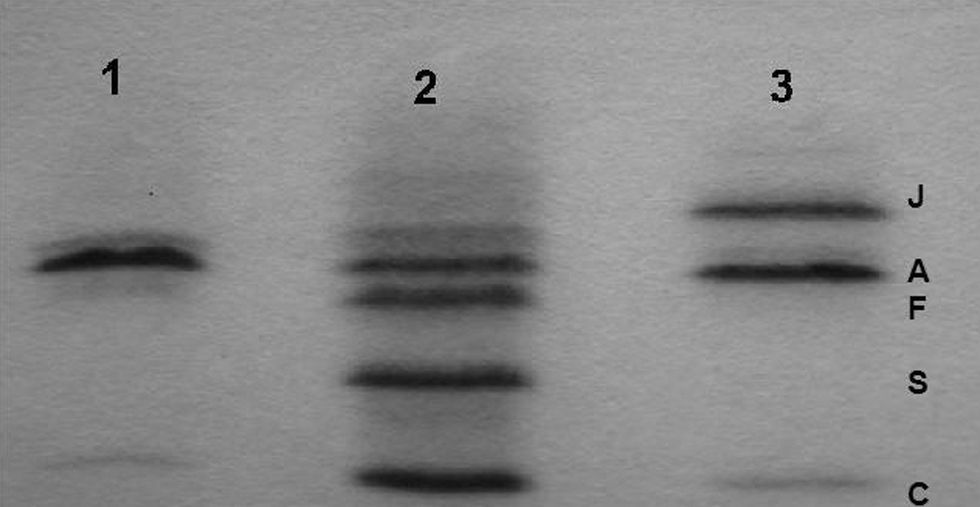
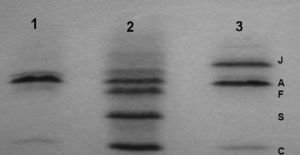
Hemoglobin study: in reverse-phase HPLC, an abnormal β chain was isolated prior to the normal β chain and cation-exchange HPLC showed an abnormal Hb. The electrophoresis on cellulose acetate at alkaline pH and isoelectric focusing (Figure 1) revealed an Hb band located at the HbJ position, faster than the HbA. In the citrate-agar electrophoresis at acid pH, the atypical Hb was not segregated from the HbA.
- •
Molecular study: a GGC→GAC mutation on codon 16 determining a change from glycine to aspartic acid in the heterozygote state was demonstrated. This variant is known as J-Baltimore [beta 16(A13) Gly>Asp].
More than 700 variants of Hb have been described to date. In most cases, there is a point mutation on one of the Hb chains, unassociated with any functional alteration. Some of these hemoglobinopathies can affect HbA1c determination by HPLC. Their incidence among diabetic individuals varies from country to country. In Europe, a prevalence of 0.06% has been described,3 whereas in Africa the prevalence is 33%.4 Some authors estimate that, in the United States, more than 150,000 diabetic persons have one of these variants.2 Some of these Hb anomalies are common and are usually associated with some type of disease, as is the case with sickle-cell anemia.5 In these cases, the interpretation of the abnormal HbA1c values is completely clear. Other hemoglobinopathies are relatively uncommon and absolutely silent, without any symptoms or complete blood count anomalies. In such circumstances, HbA1c levels that do not match capillary blood glucose levels can be difficult to explain.6 Hb J-Baltimore is one of these hemoglobinopathies. The use of other analytical methods, such as TINIA, as in our case, may enable proper determination, as these methods are not affected by these hemoglobinopathies.7 However, at most sites, HPLC is the most commonly used method, and the option of reassessing the patient's blood sample with other assays is not always readily available.
Hb J Baltimore was first described in 1963 in an African-American family. Since then, several cases have been reported in distinct racial groups. Most of these cases were discovered incidentally during the study of other entities, such as thalassemia.8 More recently, the increasingly frequent determination of HbA1c in diabetic persons has contributed to the appearance of cases of Hb J-Baltimore associated with anomalous HbA1c values.9,10 In these cases, the existence of a hemoglobinopathy must be ruled out to prevent errors in the monitoring of diabetic patients. Some authors have even recommended performing an electrophoretic study on all diabetic patients following diagnosis of diabetes.9
Given that the diagnosis of this condition depends on a specific hemoglobin study by electrophoresis, when hemoglobinopathies are suspected, most cases of Hb J will likely go undiagnosed. In diabetic patients, a lower than expected HbA1c level as per the self-monitoring blood glucose data is not an exceptional finding and may be due to several causes (undetected hypoglycemia, improper or infrequent self-monitoring, etc.). Therefore, if determination of the HbA1c does not yield a very low value (in our case, below the reference range), the possibility of hemoglobinopathy will not be considered and will be undiagnosed. Moreover, clinics routinely trust HbA1c levels more than self-monitoring blood glucose levels when making therapeutic decisions. As a result, there is an evident risk in these situations of improper treatment adjustments. For all of these reasons, we believe that, in cases of persistent and inexplicable discordance between HbA1c and self-monitoring blood glucose levels, an electrophoretic hemoglobin study should be performed.
Given the hereditary nature of both these hemoglobinopathies and type 2 diabetes mellitus, relatives must be screened to prevent potential errors in HbA1c determination if diabetes mellitus develops in the future. By taking this step, we were able to alert the daughter in our index case that her HbA1c determinations by HPLC did not reflect her actual blood glucose levels.