In Spain medullary thyroid carcinoma (MTC) would not exceed 80 new cases per year and less than half of them would be good candidates for systemic treatment with novel agents.
MethodsRelevant literature was reviewed, including PubMed searches supplemented with additional articles.
ResultsThe consensus summarizes the clinical outcomes in terms of activity and toxicity of each of the available drugs. A brief summary of the minimum requirements in terms of follow up and genetic counseling around MTC is also included.
ConclusionsOnly those patients with objective imaging progression in the last 12–14 months with large volume of disease are clear candidates to start systemic treatment. However, those patients with low disease volume should be considered for ‘wait and see’ strategy until symptoms of the disease appear. Multidisciplinary approach for the management of MTC patient is mandatory nowadays.
Se calcula que la incidencia de cáncer medular de tiroides (MTC) en España no supera los 80 nuevos casos por año y menos de la mitad podrán ser buenos candidatos para recibir tratamiento sistémico con nuevas terapias.
MétodosSe ha revisado la información científica pertinente a través de búsquedas en PubMed y otras fuentes adicionales.
ResultadosEste consenso compendia los resultados clínicos en términos de actividad y toxicidad de los fármacos actualmente disponibles. También se aborda un breve resumen con los requerimientos mínimos para el seguimiento y el consejo genético en el CMT.
ConclusionesLos pacientes candidatos para iniciar tratamientos sistémicos son únicamente aquellos con gran carga tumoral en los que se objetiva, mediante pruebas de imagen, una progresión en los últimos 12-14 meses. En aquellos pacientes con escasa carga de enfermedad se debe considerar la observación hasta que aparezcan síntomas de enfermedad. Hoy en día es preceptivo manejar al paciente con MTC por un equipo multidisciplinar.
Medullary thyroid carcinoma (MTC) is an uncommon neuroendocrine tumor that accounts for 3–5% of all thyroid gland cancers. Despite its rarity, MTC is one of the best-characterized solid tumors in terms of pathologic, biochemical, and genetic properties. MTC can be sporadic or part of several familial syndromes. Sporadic cases are developed in individual patients with no family history of MTC and account for around 75% of all MTC. Familial cases are autosomal dominant inherited. Thus, the cases can be traced from the proband along first-degree relatives. Classically familial syndromes include familial-MTC and multiple endocrine neoplasia 2 (MEN2). MEN2 is further divided into MEN2A (that normally associate MTC, pheochromocytoma and hyperparathyroidism) and MEN2B (that includes MTC, pheochromocytoma, Hirschsprung disease and Marfan-like phenotype among other entities).
Understanding MTC biology, clinical manifestations, treatment and prognosis is based extensively on the discovery of tumor-specific mutations and the abnormal expression of several tyrosine kinase receptors and pathways.1 Recent scientific breakthroughs have helped to better understand the management of advanced MTC.
In March 2014 a panel of experts from the Spanish Task Force Group for Orphan and Infrequent Tumors (GETHI) and from the Working Group of Thyroid Cancer of the Spanish Society of Endocrinology (SEEN) met in Barcelona and decided, after a productive discussion, to elaborate this consensus. Dr. Grande and Dr. Galofré were commissioned to write the manuscript that was afterwards reviewed and approved by all the experts. This consensus is focused on the incidence, diagnosis, follow up, prognosis and management of MTC patients with advanced or systemic disease.
Epidemiology of medullary thyroid carcinomaData from different series and registries show that the incidence of MTC in Europe is approximately 1500–2000 new cases per year.2 The prevalence of clinically diagnosed MCT accounts for 0.10–0.22 per 1,000,000 people; thus, it is considered a rare or uncommon disease. However, the prevalence of occult disease is higher. For instance, frequency of MTC in several autopsy series varies from 0.14 to 7%. These postmortem cases represent an indolent disease in which tumor size is virtually always sub-centimetric with no lymph node, extrathyroidal, or distant metastatic involvement.3
Fortunately the prevalence of patients with advanced or disseminated MTC, who will need systemic treatment, is much lower. It is estimated that only around 400–500 people with MTC will annually require systemic therapy in Europe.2
Extrapolating these data to our country, it can be estimated that the number of new patients diagnosed with MTC in Spain would not exceed 80 cases per year. Probably, about 30–40 of them will be candidates for systemic therapy due to the presence of advanced disease or relapse after surgery of the primary tumor.
Biology of medullary thyroid carcinomaAs mentioned, MTC is classically divided into sporadic and familial cases. The main difference between the two types is associated with the origin of the MTC-related rearranged during transfection (RET) gene mutation that generates the cancer. RET plays a central role in the pathophysiology of MTC.1,4,5 Germline RET proto-oncogene mutations are distinctive of the familial cases while mutations which occur in the sporadic cases are somatic. In other words, in familial cases RET mutation is genetically transmitted through one of the gametes. Thus, in familial MTC syndromes all the body cells (not only thyroid cells) harbor the mutated gene. On the contrary, specific RET MTC mutations only occur in the thyroid tumor cells of sporadic cases. A specific gene mutation in RET proto-oncogene is found in virtually every patient with familial MTC syndrome and in half of the cases with sporadic MTC.
Located in chromosome 10q11.2, the RET gene encodes a tyrosine kinase transmembrane receptor that is activated by GDNF (glial cell line derived neurotrophic factor). Its ligand (GDNF) belongs to the transforming growth factor-β (TGF-β) superfamily. RET is primarily expressed in cells of the neural crest and urogenital tract and is involved in cell growth, cell differentiation and cell survival.6
Preclinical studies in mice showed the responsibility of RET gene mutations in the development of MTC. These mutations can involve different exons of the tyrosine kinase receptor. Receptor mutations may involve extracellular, intracellular or even transmembrane domains. These mutations generate a constitutive activation of the cells regardless of the binding or not of the ligand (gain of function in the absence of ligand).7,8
Phenotype-genotype correlation in mutations of RET proto-oncogeneThere is some correlation between genotype and phenotype in familial MTC syndromes, and some mutated codons are linked to different syndromes. In over 98% of patients with familial cases RET mutations have been detected and around 85% are located in 609, 611, 618 and 620 codons of exon 10 and 634 codon of exon 11. Mutations in codon 634 of RET is found in nearly 80% of MEN2A patients while 10–15% harbor mutations in codons 609, 611, 618 or 620. Mutation in codon 634 is frequently associated with pheochromocytoma and hyperparathyroidism. However, 10–15% of individuals with MEN2A harbor mutations in codons 609, 611, 618 or 620. All these mutations are located in the extracellular domain. Nearly 95% of MEN 2B have point mutations in codon 918 of exon 16 and the others in codons 883 and 922.2
The mutations in sporadic MTC (found only in tumor cells) can be diverse and the list covers many different codons, such as 631, 634, 766, 768, 876, 804, 883, 884, 901, 922, and 930.6
Some mutations are associated with major aggressiveness, high probability of lymph node metastases, recurrent/persistent disease and poor survival.9 The 2009 American Thyroid Association (ATA) Guidelines established a four-category genotype–phenotype correlation and risk levels for aggressive MTC.10 The ATA levels are A to D, D being the highest risk. MEN 2B is normally associated with level D. For instance, the 10-year survival rate in patients harboring codon 918 mutations is 50%, which is poorer than that observed in patients without this mutation (approximately 85%).
Other molecular alterationsIn addition to RET mutations, MTC is influenced by other disorders of the tumor microenvironment, such as high vascularization, elevated levels of vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), hepatocyte-growth factor receptor (c-MET) and platelet-derived growth factor (PDGF). These events promote the tumor to have a high capillary micro-density allowing tumor cells to enter the blood vessels and leading to distant metastases.11
Prognosis of medullary thyroid carcinomaAt the time of diagnosis, about 50% of patients with MTC have metastasized beyond the thyroid, mainly to regional lymph nodes, and around 15% have distant metastases. About 90% of these patients with metastatic MTC will die as a consequence of disease progression.12
It has been reported that the overall survival rates at 5 and 10 years in patients with MTC ranges from 80–97% to 75–88%, respectively. Survival depends mostly on the disease stage. An early diagnosis allows complete surgical cure in patients with local disease (stages I and II). However, the presence of lymph node and distant metastases at first diagnosis significantly worsens the prognosis and survival rates in MTC. Nevertheless, in patients with distant metastases (stage IV), survival at 5 years is 40% with a median overall survival of 2–3 years.13
Age is also an important prognosis factor. Patients under 40 have a survival of 95 and 75% at 5 and 10 years respectively, whereas survival decreases from 65 to 50% in patients older than 40 years old for the same time period.
Symptoms of advanced medullary thyroid carcinomaMost of the symptoms caused by advanced disease are pain due to tumor growth and the appearance of cervical lymph nodes. Around 30–60% of MTC have palpable local lymph nodes at presentation; this is the most common site of metastases, so that the resulting symptoms will depend on their size and anatomical location. The presence of lymph nodes is related to tumor size. Infracentimetric tumors are associated with positive lymph nodes in 10–30% of the patients. However, a thorough histologic analysis discovers features of tumor involvement in 80% of cervical lymphadenopathies.1
Respiratory symptoms such as dyspnea, chest pain or recurrent respiratory infections due to the presence of lung metastases are relatively common. Similarly, bone lesions can lead to associate symptoms such as pathological fractures and pain.
In spite of being a frequent complication, milliary hepatic metastases usually cause no symptoms. Only in advanced stages of the disease liver metastases induce hepatic failure or biliary stasis.1
Although MTC is a neuroendocrine tumor, the majority of patients, including those with disseminated disease, have no symptoms derived from peptide hormone secretion. Rarely MTC can manifest itself by the effects of active peptides such as ACTH (generating Cushing's syndrome) or calcitonin gene-related peptide that produces facial flushing and diarrhea.1
Tumor markers at diagnosis and follow up for medullary thyroid cancer: are calcitonin and carcinoembryonic antigen (CEA) reliable tumor markers?It is well established that calcitonin and CEA are the current tumor markers for MTC. These two markers, and especially calcitonin, help in the disease assessment during follow-up. Unfortunately there is no serum calcitonin cutoff value that clearly discriminates between benign and malignant cases. It should be borne in mind that, although very rarely, some cases of MCT with normal calcitonin values have also been described.14
Additionally, the assessment of tumor extension by imaging techniques such as neck ultrasound and whole body computed tomography (CT) scan is mandatory. Ultrasound of the cervical region has a very high sensitivity to evaluate the presence of the primary tumor (and of residual or recurrent disease during follow-up) as well as or regional lymph node involvement.
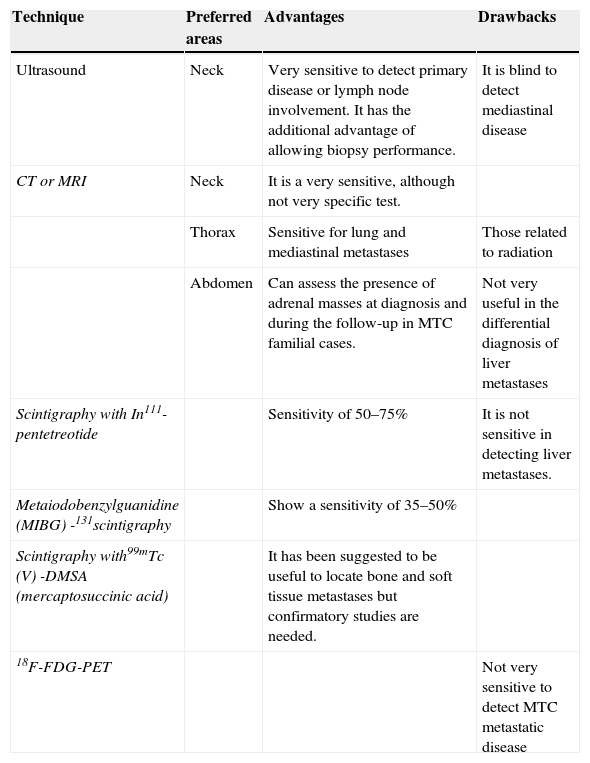
Imaging tests at diagnosis to evaluate the presence of distant metastases (usual target organs are liver, lungs and bone) include CT scan or magnetic resonance imaging (MRI) of the neck, thorax and abdomen. Liver metastases are hyperechoic on ultrasound and may be confused with hemangiomas. Abdominal imaging techniques may also help to rule out (in case of biochemical backup) the presence of pheochromocytoma in those patients harboring RET germline mutation. Bone metastases can also be diagnosed with bone scintigraphy, although a large number of these patients have lytic metastases that are difficult to evaluate by nuclear medicine tests. Head CT or MRI is not indicated unless the presence of brain metastases is suspected. Fluorodeoxyglucose (FDG)-Positron emission tomography (PET) is not very useful in this setting due to MTC low uptake (Standardized Uptake Value lower than 5), except for tumors that have a more aggressive clinical behavior or growth15 (Table 1).
Imaging methods available for MTC monitoring.
| Technique | Preferred areas | Advantages | Drawbacks |
|---|---|---|---|
| Ultrasound | Neck | Very sensitive to detect primary disease or lymph node involvement. It has the additional advantage of allowing biopsy performance. | It is blind to detect mediastinal disease |
| CT or MRI | Neck | It is a very sensitive, although not very specific test. | |
| Thorax | Sensitive for lung and mediastinal metastases | Those related to radiation | |
| Abdomen | Can assess the presence of adrenal masses at diagnosis and during the follow-up in MTC familial cases. | Not very useful in the differential diagnosis of liver metastases | |
| Scintigraphy with In111-pentetreotide | Sensitivity of 50–75% | It is not sensitive in detecting liver metastases. | |
| Metaiodobenzylguanidine (MIBG) -131scintigraphy | Show a sensitivity of 35–50% | ||
| Scintigraphy with99mTc (V) -DMSA (mercaptosuccinic acid) | It has been suggested to be useful to locate bone and soft tissue metastases but confirmatory studies are needed. | ||
| 18F-FDG-PET | Not very sensitive to detect MTC metastatic disease |
Calcitonin is the most reliable tumor marker at diagnosis and in the follow up for monitoring and evaluation of treatment efficacy in patients with MTC.16
Basal serum calcitonin levelElevated calcitonin level correlates with MTC tumor size, C cell hyperplasia and also with the extent of metastases.17
Calcitonin during follow-upA high postoperative circulating calcitonin value is the best predictive factor for biochemical recurrence. In general, 1cm3 of MTC primary tumor is associated with a serum calcitonin level of approximately 1000pg/mL. Postsurgical calcitonin levels around 10–150pg/mL are indicative of locoregional disease. Distant metastases and extra thyroidal disease generally occur in patients with basal calcitonin higher than 150pg/mL. Therefore, baseline presurgical calcitonin levels help to individualize the extent of the surgery and postsurgical serum levels orientate the postoperative follow up.18
Carcinoembryonic antigen (CEA)Basal serum CEA levelCEA is a less sensitive and less specific tumor marker for diagnosis, but it is also useful in follow up. CEA levels are usually associated with distant disease as well as tumor clinical activity. CEA has a very low positive predictive value, presenting high values in other unrelated conditions such as gastrointestinal and gynecological neoplasms, smokers and it has been associated with the intake of certain foods.
Markers doubling timeIn MTC patients, the doubling time of both calcitonin and CEA are efficient tools for assessing postoperative tumor burden and progression.19 Furthermore, there is a clear relationship between the doubling time of calcitonin and overall survival.
A calcitonin doubling time of less than two years is generally associated with poor prognosis.6 Patients with a doubling time of calcitonin of less than six months have an overall survival around two years. However, patients with a doubling time of calcitonin ranging between six and 24 months have an eight year overall survival.
An analysis of a large series shows that the rate of patients with radiographic or symptomatic disease progression at one year is 94% if the doubling time of calcitonin is less than two years. Conversely, if the calcitonin doubling time is more than two years 86% of patients present stable disease after one year of follow up.15
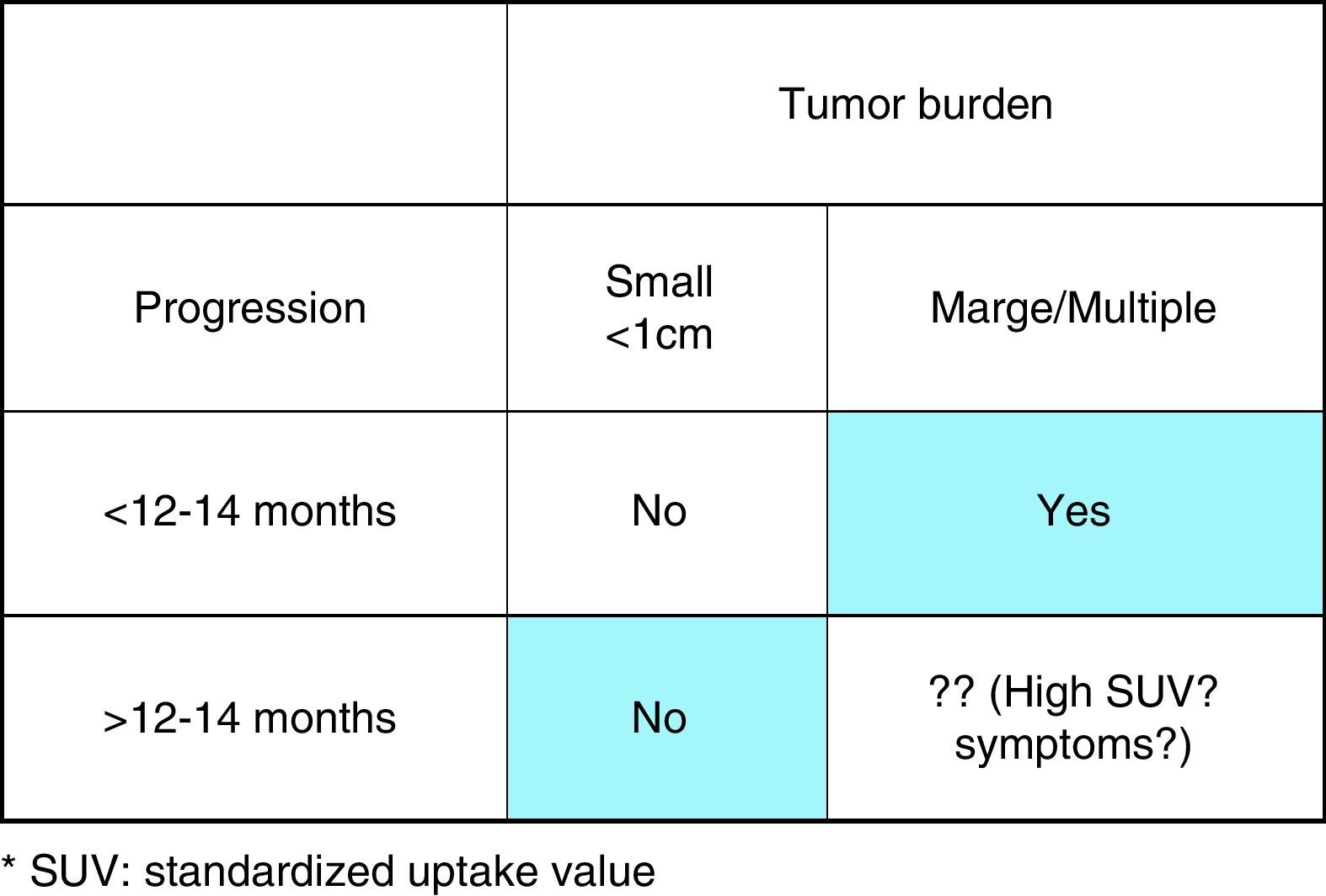
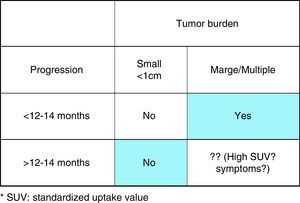
When and how should metastatic disease be treated?The presence of metastatic disease at diagnosis does not warrant on its own the initiation of systemic treatment. In general, systemic treatment should only be offered to patients with measurable (by Response Evaluation Criteria in Solid Tumors, RECIST criteria) metastatic disease, and/or symptoms produced by the disease itself.20 Systemic treatment should not be offered to patients with disseminated MTC with only: (a) High levels of calcitonin and no macroscopic structural disease, (b) Low tumor burden; and (c) Absence of macroscopic progression (assessed by imaging techniques).
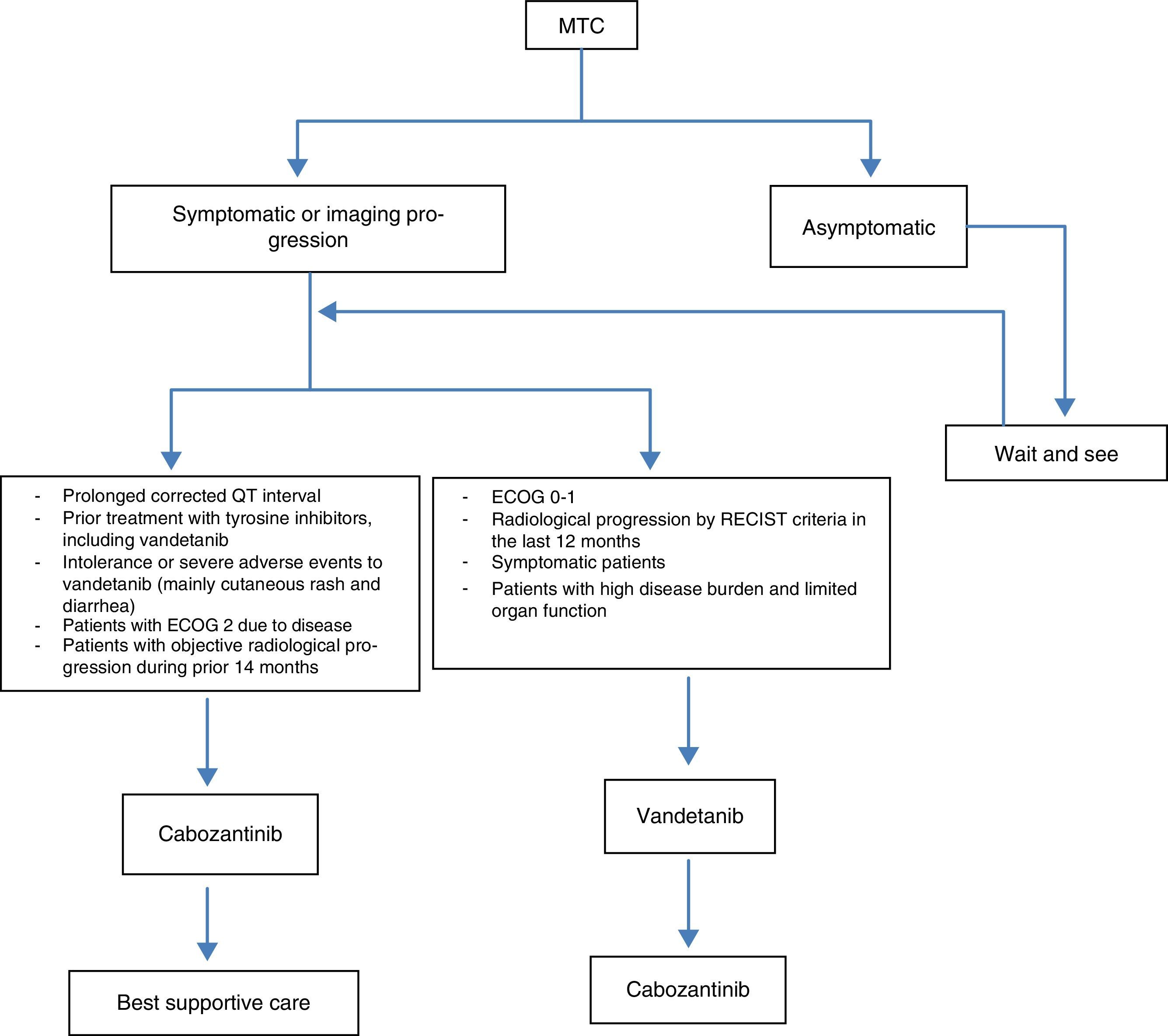
Initiation of systemic therapy in patients with advanced medullary thyroid carcinoma (Fig. 1)Active treatment is most often indicated for patients with lesions in critical locations such as brain and lung metastases, presence of symptomatic metastases, hormonal secretion or active fracture of a weight bearing bone.10 Therefore, the goals of systemic therapy are: (a) to provide locoregional disease control; (b) palliate symptoms of excess of hormonal production (such as diarrhea, Cushing's syndrome, etc.); (c) alleviate the symptoms of metastases (pain) and (d) control distant metastases that may cause collateral harm.
Advanced medullary thyroid carcinoma criteria to systemic treatment onset. Only those patients with objective imaging progression in the last 12–14 months and with large volume of disease are clear candidates to start systemic treatment. However, those patients with low volume of the disease should be considered for ‘wait and see’ strategy until symptoms of the disease are appearing.
Surgery is the mainstay of treatment for MTC and the overwhelming majority of patients who undergo complete resection of their localized disease will do well. However, the role of surgery in disseminated disease is more limited and controversial. In most cases, surgery is considered as a way to reduce tumor size (debulking), alleviate symptoms and prevent the onset of complications.
In general, due to the frequent milliary hepatic disease dissemination, surgery of liver metastases secondary to MTC is not recommended. Any indication for surgery in advanced disease must be agreed by a multidisciplinary team of thyroid cancer specialists.21
RadiotherapyCurrently there are no clear indications for external beam radiation therapy (EBRT). It could be locally indicated in inoperable patients as well as in those patients that maintain high levels of calcitonin after surgery. MTC is relatively resistant to radiation therapy and the scientific evidence for the use of EBRT in this setting is limited.22
Novel tyrosine kinase inhibitorsMost of the new treatments currently available and most of the new drugs in clinical development against MTC have tyrosine-kinase receptors as a target.
To date, vandetanib and cabozantinib are two multikinase inhibitors that have been proven to be effective in randomized phase III trials, and their use has been approved by most of the international regulatory agencies (Table 2a).
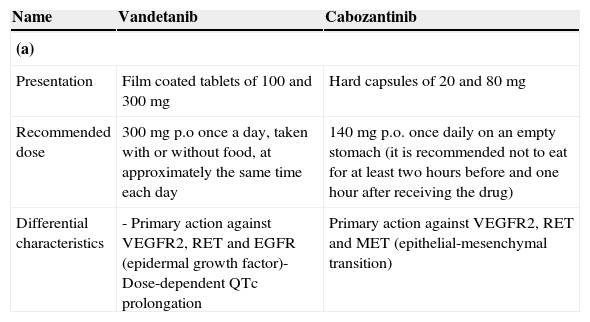
(a) Vandetanib and cabozantinib: main pharmacological characteristics; (b) EXAM and ZETA studies: indirect comparison; (c) EXAM and ZETA studies: comparison of grade 3 or 4 adverse events and (d) cabozantinib vs. vandetanib: safety and tolerability.
| Name | Vandetanib | Cabozantinib |
|---|---|---|
| (a) | ||
| Presentation | Film coated tablets of 100 and 300mg | Hard capsules of 20 and 80mg |
| Recommended dose | 300mg p.o once a day, taken with or without food, at approximately the same time each day | 140mg p.o. once daily on an empty stomach (it is recommended not to eat for at least two hours before and one hour after receiving the drug) |
| Differential characteristics | - Primary action against VEGFR2, RET and EGFR (epidermal growth factor)- Dose-dependent QTc prolongation | Primary action against VEGFR2, RET and MET (epithelial-mesenchymal transition) |
| Clinical trial and patient population | Sponsor | Study description | Efficacy data | Safety data |
|---|---|---|---|---|
| (b) | ||||
| EXAMPhase IIINCT00704730MTC patients with unresectable locally advanced progression or metastatic disease (patients with brain metastases were excluded)ECOG≤2 | Exelixis | Treatment: cabozantinib (140mg) vs. placeboNumber of patients: 330 randomized 2: 1 to placebo or cabozantinibMain end-point: PFSSecondary end-points: OS, ORRR, safety and tolerability | Cabozantinib (n=219) vs. placebo (n=111):PFS: 11.2 vs. 4.0 months (HR 0.28, 95% CI 0.19–0.40, P<0.0001).The study met primary objective ORRR: 28% vs. 0% (P<0.0001). OS at interim analysis (44% of the required events): no significant difference between the two study arms | Grade ≥3 adverse events (cabozantinib vs. placebo):Diarrhea: 15.9% vs. 1.8%Hand foot syndrome: 12.6% vs. 0%Fatigue: 9.3% vs. 2.8%Hypocalcemia: 9.3 vs. 0%Hypertension: 7.9 vs. 0%Dose reduction: 79% of the patients |
| ZETAPhase IIINCT00410761MTC patients with unresectable locally advanced or metastatic disease | AstraZeneca | Treatment: vandetanib (300mg once daily) or placeboNumber of patients: 331 (vandetanib n=231, placebo n=100)Main end-point: PFS by RECISTSecondary end-points: ORRR, disease control rate and OS | Median PFS:Vandetanib estimated at 30 months (not reached) Placebo: 19.3 monthsORRR:Vandetanib: 45%Placebo: 13%Rate of disease controlVandetanib: 87%Placebo: 71%OS: no significant difference between the two study arms | Grade ≥3 adverse events (vandetanib vs. placebo):Diarrhea: 11% vs. 2%Hypertension: 9% vs. 0%Prolonged corrected EKG: 8% vs. 1%Fatigue: 6% vs. 1%Dose reduction: 35% of patients |
| EXAM and ZETA studies: comparison of grade 3 or 4 adverse events | EXAM study cabozantinib | EXAM study placebo | ZETA study vandetanib | ZETA study placebo |
|---|---|---|---|---|
| (c) | ||||
| Diarrhea | 34 (16%) | 2 (2%) | 25 (11%) | 2 (2%) |
| Hand foot syndrome | 27 (13%) | 0 | NA | NA |
| Fatigue | 20 (9%) | 3 (3%) | 13 (6%) | 1 (1%) |
| Hypertension | 18 (8%) | 1 (1%) | 20 (9%) | 0 |
| Prolonged QTc | NA | NA | 18 (8%) | 1 (1%) |
| Asthenia | 12 (6%) | 2 (2%) | 6 (3%) | 1 (1%) |
| Weight loss | 10 (5%) | 0 | 20 (9%) | 0 |
| Loss of appetite | 10 (5%) | 1 (1%) | 9 (4%) | 0 |
| Cutaneous rash | 2 (1%) | 0 | 8 (4%) | 1 (1%) |
| Variable | EXAM study (Cabozantinib) (%) | ZETA study (Vandetanib) (%) |
|---|---|---|
| (d) | ||
| Grade 5 adverse events (death) | 5 | 2 |
| Percentage of patients that require dose reduction vs. placebo | 79 vs. 9 | 35 vs. 3 |
| Percentage of withdrawals due to adverse events | 22 | 8 |
| Percentage of patients with Grade 3 or greater adverse events | 69 | 47 |
| Percentage of patients with no progression after one year in the placebo group | 7 | 63 |
PFS: progression-free survival; OS: overall survival; ORRR: objective radiologic response rate.
Vandetanib is a tyrosine kinase inhibitor that acts on VEGFR 2, VEGFR-3, RET, and epidermal-growth factor receptor (EGFR). Vandetanib was one of the first drugs that showed activity in cell lines of thyroid cancer, mainly through its action on RET/PTC rearrangements and mutations in the RET gene. Vandetanib was the first drug approved by the American Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the treatment of patients with advanced MTC.23–25
The ZETA study is a phase III, double-blind clinical trial with a 2:1 randomization, in which patients with sporadic or hereditary MTC were randomized to receive either vandetanib (300mg) or placebo daily26 (Table 2b). The median progression-free survival for patients treated with vandetanib was significantly higher than the placebo-treated arm (19.3 months in the placebo arm vs. median not reached in the vandetanib arm, HR: 0.46; 95% CI: 0.31–0.69, p<0.001, after a follow up of 24 months). Patients receiving vandetanib also obtained benefits in terms of response rate and rate of disease control, compared to controlled arm. The objective radiologic response rate was 45% in the vandetanib arm versus 13% in the placebo arm (p<0.001), while the rate of disease control was 87% in the vandetanib arm versus 71% of the placebo arm (p=0.001). All patient subgroups analysis, including gender, performance status at start of treatment, initial disease stage, number of previous treatments and treatment response, showed a significant higher benefit achieved by the vandetanib arm. Such benefits were observed both in patients with sporadic as well as hereditary disease. However, due to the low number of patients with sporadic MTC and negative RET mutation and the elevated number of patients with unknown RET mutation status definitive conclusions about the benefit of vandetanib based on RET mutational status could not be drawn.26
CabozantinibCabozantinib inhibits various membrane receptors including VEGFR-2, MET receptor and the RET receptor.27 The EXAM study is a randomized phase III, double-blind testing the efficacy of cabozantinib against placebo. The results were significant for the main end-point of the study, that is, progression-free survival (11.2 vs. 4 months, HR 0.28, 95% CI 0.19–0.40, P<0.001, for cabozantinib vs. placebo, respectively).28 The trial allowed patient enrollment regardless of age or compromised condition (up to 10% of patients had ECOG 2), and a large proportion of patients (39%) had received at least one prior systemic therapy based on chemotherapy, tyrosine kinase inhibitors or somatostatin analogs. Within the EXAM study cabozantinib showed a reduced risk of progression of 52% compared to placebo in the subgroup of patients who had received prior treatment with another tyrosine kinase inhibitor. Table 2a–d compares the clinical outcome of vandetanib and cabozantinib in terms of activity, safety and tolerability.
Role of chemotherapyTraditional chemotherapy has not achieved great results in the treatment of patients with advanced MTC. This could be due to the lack of rigorous recent studies. The earliest studies were conducted over 30 years ago, with different assessment methods from the current ones to evaluate response.
The cytotoxic agent that has been most commonly used in the treatment of advanced MTC is doxorubicin. This drug achieves a radiological objective response rate assessed by RECIST criteria ranging from 0 to 22%. Unfortunately, the limited responses achieved are partial responses, having a very short duration, and are associated to high toxicity. The use of standard chemotherapeutic agents should not be considered as first-line therapy for patients with persistent or recurrent MTC.29
Treatment of symptoms associated to hormonally active metastasisDiarrheaDiarrhea therapy should begin with antimotility agents and alternatively, somatostatin analogs. Aggressive local therapies such as surgical debulking or transarterial hepatic chemoembolization may be considered in selected patients.10
Cushing's syndromeMTC patients with Cushing's syndrome typically have poor prognosis. Treatment should be considered even in the setting of widely metastatic MTC because the syndrome can be severe and debilitating. Cushing's syndrome may be treated in a multimodality manner with medical therapy directed toward the hypersecretion of cortisol, or even bilateral adrenalectomy (which is, probably, the best option in many cases10).
Treatment according to metastases locationNeck recurrenceReoperation is the main treatment for local or regional recurrence in the neck or mediastinum and surgery can be followed by external radiation therapy when neck involvement is extensive. However, a complete biochemically evident cure is rarely achieved.14
Brain metastasesPatients with isolated or limited brain metastases should be considered for surgical resection. EBRT may be indicated for brain metastases not amenable to surgery.10
Bone metastasesTreatment options involve surgery, EBRT, embolization, cement injection and bisphosphonates depending on the location and extent of the metastases.14
Liver metastasesThe hepatic transarterial chemoembolization may be considered in patients with progressive liver metastases and should preferably be performed at an early stage. Partial responses may be achieved in around 40% of patients.30
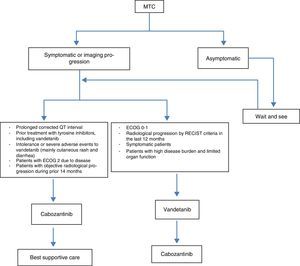
Management of advanced medullary thyroid carcinoma: multidisciplinary consensus algorithmFig. 2 summarizes the multidisciplinary consensus algorithm for the management of advanced MTC.
Factors that modulate medullary thyroid carcinoma prognosisOverall MTC prognosis is intermediate between differentiated and undifferentiated thyroid carcinoma. The clinical course of patients with MTC is variable, even in the subgroup of patients submitted to surgery with curative intent and with persistent elevated calcitonin levels postoperatively.
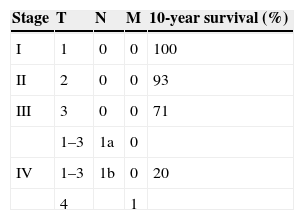
The 10-year disease-free and survival-rate of MTC is approximately 75%. Important prognostic factors that predict adverse outcome include advanced age at diagnosis, extent of primary tumor, nodal disease, and distant metastasis. Using a previous TNM classification system, 10-year survival rates for stages I, II, III and IV are: 100%, 93%, 71%, and 21%, respectively (Table 3). The presence of distant metastases indicates a 25% survival rate at five years and 10% at 10 years. Distant metastases constitute the main cause of death related to MTC. Metastases may involve multiple organs such lungs, bones and liver, and more rarely the brain, skin, and breast.
10-year survival according to TNM classification.a
| Stage | T | N | M | 10-year survival (%) |
|---|---|---|---|---|
| I | 1 | 0 | 0 | 100 |
| II | 2 | 0 | 0 | 93 |
| III | 3 | 0 | 0 | 71 |
| 1–3 | 1a | 0 | ||
| IV | 1–3 | 1b | 0 | 20 |
| 4 | 1 |
The behavior of patients with residual disease varies widely and depends largely on the initial extent of disease. Morbidity in MEN2 cases is mainly conditioned by pheochromocytoma.
The presence of a somatic RET mutation correlates with a worse outcome in sporadic MTC patients, not only for the higher probability of persistent disease, but also for a lower survival rate on a long-term follow up. More interestingly, the presence of a somatic RET mutation correlates with the finding of lymph node metastases at diagnosis, which is a known bad prognostic factor for the definitive cure of MTC patients.5
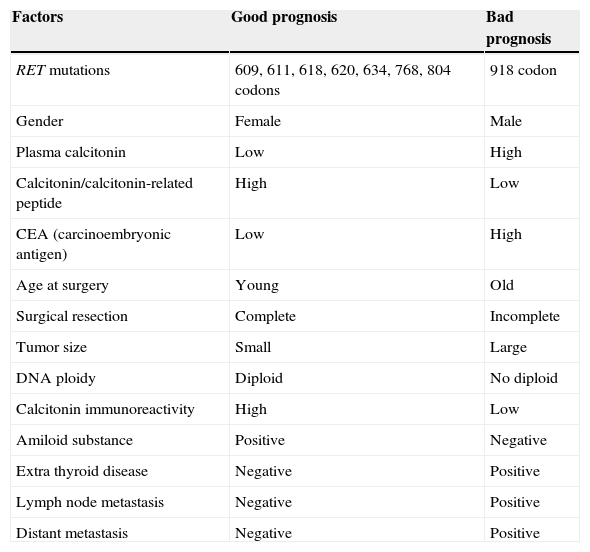
The most important prognostic factors are listed in Table 4.
Prognostic factors.
| Factors | Good prognosis | Bad prognosis |
|---|---|---|
| RET mutations | 609, 611, 618, 620, 634, 768, 804 codons | 918 codon |
| Gender | Female | Male |
| Plasma calcitonin | Low | High |
| Calcitonin/calcitonin-related peptide | High | Low |
| CEA (carcinoembryonic antigen) | Low | High |
| Age at surgery | Young | Old |
| Surgical resection | Complete | Incomplete |
| Tumor size | Small | Large |
| DNA ploidy | Diploid | No diploid |
| Calcitonin immunoreactivity | High | Low |
| Amiloid substance | Positive | Negative |
| Extra thyroid disease | Negative | Positive |
| Lymph node metastasis | Negative | Positive |
| Distant metastasis | Negative | Positive |
Due to its biological, pathological, serological, imaging, and clinical peculiarities, advanced MTC should be handled in the context of a multidisciplinary team where endocrinologists, pathologists, clinical biochemists, radiologists, radiation oncologists, head and neck surgeons and medical oncologists should work together to share their specific knowledge in order to achieve the optimal patient outcome.
In the last few years, advanced MTC has moved from being a disease orphan of treatment to have several systemic drugs available for management. However, the cure of patients with advanced disease is rarely achieved and always needs the intervention of surgery. It is therefore necessary to expand our knowledge in order to complete a better understanding of the molecular biology that underlies this tumor as well as the mechanisms of resistance to the currently available drugs. Nowadays, overall survival remains limited despite the addition of tyrosine kinase inhibitors like vandetanib and cabozantinib to our therapeutic repertoire.
In addition, many questions remain unanswered such as: when should treatment be started, how to detect the presence of disease in patients that only show elevated circulating tumor marker, which is the best systemic treatment available or how the studies should be best sequenced.
There are also many challenges such as tailoring the targeted molecular biomarkers to help determine which tumors might do better with the available treatments, as well as the need of finding more accurate and sensitive techniques that allow to detect the presence of micrometastases, among others.
The greater advances will be achieved by working together across disciplines. In rare tumors overall, and in the case of MTC in particular, it is critical to create centers specialized in their management.
Conflict of interestJCG is a consultant or speaker or has received honoraria within the past 12 months from AstraZeneca, Bayer, Genzyme-Sanofi and Merck-Serono.