The upper limit of TSH reference level is controversial. The purpose of our study was to determine TSH reference values in a Mexican population in accordance with the National Academy of Clinical Biochemistry (NACB) criteria and in correlation with thyroid ultrasound (US) examination.
Patients and methodsThe study was conducted in volunteers with no known thyroid disease. We recruited 482 subjects, most of them medical or administrative staff from our hospital. They answered a questionnaire on demographic data, family history, co-morbidities, and drug use. Their thyroid hormone levels and thyroid antibodies were determined, and a complete physical examination and thyroid US were performed. The population used to establish the TSH reference intervals was selected according to the NACB criteria and their normal thyroid structural and echogenic characteristics in US examination.
ResultsAmong 482 subjects (209 males) with a median age of 26 years, prevalence rates of TPOAb and TgAb were 9.3% and 10.3% respectively. Mean TSH level in the overall population was 1.90±1.94, with a 97.5th percentile of 6.76mIU/L. The reference population was limited to 282 subjects (41.5% were excluded) with a mean TSH of 1.86±1.63 and a 97.5th percentile of 4.88mIU/L. No sex difference was found (p=0.287). Median urinary iodine level in the reference population was 267μg/L IQR (161.3–482.5).
ConclusionsThe TSH reference interval in the reference population was 0.71 (CI 0.65–0.77) to 4.88mIU/L (CI 4.5–5.3); these limits may be influenced by iodine nutritional status in this population.
Existe controversia respecto al límite superior de referencia para TSH. El objetivo del estudio fue determinar los valores de referencia para TSH en una población mexicana de acuerdo con los criterios de la National Academy of Clinical Biochemistry (NACB) y en correlación con el examen ultrasonográfico (US) tiroideo.
Pacientes y métodosEl estudio se realizó en voluntarios sin enfermedad tiroidea conocida. Se reclutaron 482 individuos, personal sanitario y administrativo del hospital, que respondieron un cuestionario sobre datos demográficos, antecedentes familiares, co-morbilidades y medicamentos consumidos, y a los que se les practicó determinación de hormonas tiroideas, anticuerpos anti-tiroideos, exploración y US tiroideo. La población escogida para establecer los intervalos de referencia de TSH fue seleccionada con los criterios de la NACB más la normalidad estructural y ecogénica del tiroides por US.
ResultadosEn los 482 sujetos (209 hombres) con mediana de edad de 26 años, la prevalencia de TPOAb fue de 9,3% y TgAb 10,3%. La media de TSH para la población total fue 1,90±1,94, con percentil 97,5 de 6,76 mUI/L. La población de referencia se limitó a 282 sujetos (41,5% fueron excluidos); la TSH media de esta población fue de 1,86±1,63, con percentil 97,5 de 4,88 mUI/L, sin diferencia entre géneros (p=0,287). La mediana para la yoduria de la población referencia fue 267μg/L RIQ (161,3-482,5).
ConclusionesEl intervalo de referencia para la TSH fue de 0,71 (IC 0,65-0,77) a 4,88 mUI/L (IC 4,5-5,3); el resultado posiblemente está influido por el estado nutricional de yodo de esta población.
An intense debate over the upper TSH reference limit in adults1–3 has taken place in the past few years. This resulted because of a publication by the National Academy of Clinical Biochemistry (NACB) that suggested decreasing it to 2.5mIU/L.4 As with any reference value, this is pivotal in daily clinical practice. The prevalence of spontaneous hypothyroidism is 1–2% in populations with sufficient iodine intake but it increases in the elderly, especially up to 10-fold in females; moreover, the prevalence of subclinical hypothyroidism ranges between 4.0 and 8.5%.5 The NACB also suggested that in order to establish TSH reference intervals, these should be determined on the basis of 95% confidence intervals of the transformed logarithmic values obtained, in at least 120 normal volunteers rigorously selected for their euthyroid status and with no detectable antithyroid antibodies, no personal or family history of thyroid disease, a normal thyroid gland on examination and no drug intake other than estrogen.4 Ultrasound has proved useful as a screening tool because abnormal echogenicity correlates with thyroid autoimmunity,6,7 so that ultrasonographic evaluation has been added to the NACB criteria in several studies determining TSH reference intervals.8,9 Most studies establishing TSH reference intervals in adult populations in accordance with the NACB criteria have been conducted in developed countries. In Mexico there is no information on TSH reference intervals in healthy adult populations or studies based on the NCAB criteria.
The purpose of this study was to determine TSH reference intervals in a normal healthy population following the NACB's guidelines in association with ultrasound thyroid screening.
Materials and methodsThis study was approved by the Ethics Committee of the Instituto Nacional de Ciencias Médicas y Nutrición “Salvador Zubirán”.
This cross-sectional study included healthy adults over the age of 18 with no previous history of thyroid disease; they were all recruited among the hospital's administrative personnel, nurses, and physicians (residents and undergraduate students). All participants signed an informed consent form for enrollment. All the volunteers filled out a questionnaire on their family history of thyroid disease, personal pathological history and co-morbidities, as well as the use of drugs and contrast material for radiological studies. Thyroid ultrasound was performed with a portable unit (7.5MHz lineal transducer) by a single investigator (AFR), an endocrinologist with experience in thyroid sonography. This evaluation took place before thyroid examination and the operator had no access to each volunteer's information. The volume of each thyroid lobe was calculated with the following formula (mL): width×height×length×0.52; the sum of both lobes yields the total thyroid volume.7 Ultrasound gain was adjusted so as to keep operating conditions constant and until the internal jugular vein and carotid artery lumens were free of echoes. Normal or decreased echogenicity was determined by comparison with the surrounding neck muscles; thyroid hypoechogenicity was graded as 1 or mild, 2 or moderate, and 3 or marked, this latter referred to echogenicity similar to that of the sternocleidomastoid muscle.10 Echogenicity was defined as decreased only if it was diffuse and it involved the entire gland; focal echogenic compromise was ignored.
Nodular changes over 5mm were considered thyroid nodules and classified as single or multiple, predominantly solid or cystic. There are no reports in the literature on the thyroid volume reference values in adult Mexican populations, so we established a diagnosis of goiter if the thyroid volume exceeded the 97.5th percentile of the data distribution.
All volunteers were examined by the same experienced endocrinologist. A venous blood sample was collected from all volunteers after at least 8 hours’ fast, between 0700 and 1000 am; after centrifuging, the serum was aliquoted and frozen at −20°C until processing. Thyroid dysfunction was defined according to the following criteria: clinical hypothyroidism was diagnosed if the TSH was >4.0mIU/L and FT4<9.0pmol/L; subclinical hypothyroidism was diagnosed with a TSH>4.0mUI/L and FT4 between 9.0 and 23.2pmol/L; clinical hyperthyroidism was diagnosed with a TSH<0.3mIU/L and FT4>23.2pmol/L and/or TT3>2.9nmol/L; subclinical hyperthyroidism was diagnosed with a TSH<0.3mIU/L, FT4 between 9.0 and 23.2pmol/L, and TT3 between 0.9 and 2.9nmol/L.
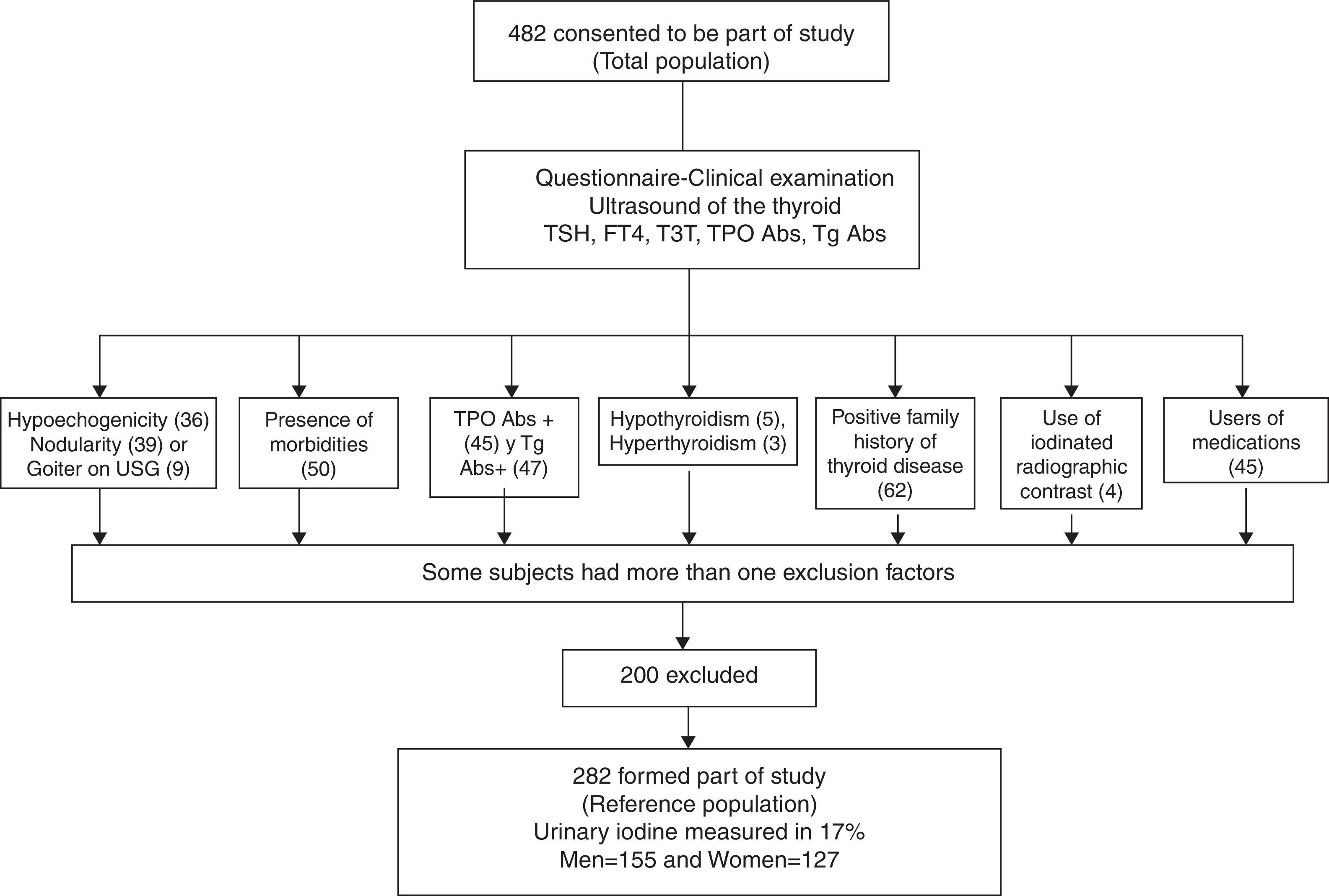
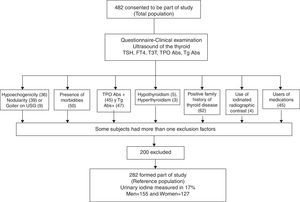
With this sampling, we defined our reference population according to the following exclusion criteria (Fig. 1): no antithyroperoxidase (TPOAb) or antithyroglobulin (TgAb) antibodies, no personal or family history of thyroid dysfunction, no goiter (either clinical or detected by ultrasound), no drug use (other than estrogen), no thyroid structural abnormalities by ultrasound, no nodules or grade 3 or marked hypoechogenicity, and no clinical dysthyroidism. Only grade 3 hypoechogenicity was an exclusion criterion, because it correlates best with lymphocytic thyroiditis diagnosed by cytology.6
The TSH results in this group were used to determine the reference value in this adult Mexican population. Urinary iodine concentrations were only determined in 48 volunteers in the reference population; they were chosen by simple randomization and their resulting ioduria defined the reference population's nutritional status.
Laboratory evaluationSerum-free T4 (FT4), total T3 (TT3), TPOAb and TgAb were measured by radioimmunoassay (RIA). FT4 had an analytical sensitivity of 0.65pmol/L and a normal range between 9.0 and 23.2pmol/L; intra-assay coefficient variation (CV) was <8.15%, and interassay CV was <14.9%. TT3 had an analytical sensitivity of 0.15nmol/L and a normal range between 0.9 and 2.9nmol/L, an intra-assay CV<12.0%, and an interassay CV<10.1%. TgAb had an analytical sensitivity of 2.0IU/mL, and values below 30IU/mL were considered normal; the intra-assay CV was <8.3%, and the interassay CV was <12.8% (RIA-gnost® FT4, RIA-gnost® T3, TGAB ONE STEP®; Cisbio Bioassays, France). The assay used for TPOAb had an analytical sensitivity of 1.9IU/mL, and values below 100IU/mL were considered normal; intra-assay CV was <9.31%, and interassay CV was 12.4% (Anti-hTPO RIA KIT® Izotop, Budapest, Hungary). TSH was determined by immunoradiometric assay (IRMA) with an analytical sensitivity of 0.005mIU/L and a normal range between 0.3 and 4.0mIU/L; the intra-assay CV was <4%, and the inter-assay CV was 3.5% (Turbo TSH 125I IRMA KIT® Izotop, Budapest, Hungary).
Urinary iodine was measured with a microplate-based kinetic method based on Sandell–Kolthoff's reaction, with results expressed in μg/L; this was conducted in the Nutritional Biochemistry Laboratory of the Instituto de Nutrición de CentroAmérica y Panamá (INCAP)/International Resource Laboratories for Iodine (IRLI)-Guatemala.
Statistical analysisCategorical variables are presented as frequencies and proportions. The distribution of continuous numerical variables was determined with the Kolmogorov–Smirnov and Shapiro–Wilk tests; those with an abnormal distribution are shown as medians and interquartile ranges (IQRs).
TSH and thyroid hormone reference values were calculated after logarithmic transformation and outlier elimination with the conventional test ±3 SD; to confirm the normal distribution after the logarithmic transformation, Anderson–Darling's test was used. The central 95% range between the 2.5th and the 97.5th percentiles was taken to be the reference interval, and the two-sided, non-parametric 0.90 confidence intervals were calculated for the lower as well as upper reference values.11 Calculation of reference values was done with CBstat 5.1.2. Software (K. Linnet. Charlottenlund, Denmark).
Group comparisons were analyzed with Student's independent t test, Fisher's exact test, and Mann–Whitney's U test, as appropriate. In all statistical analyses, p<0.05 was considered statistically significant. The SPSS 15.0 statistical software package was used (Chicago, IL, USA).
ResultsFour hundred and eighty-two (482) medical and non-medical personnel from the Instituto Nacional de Ciencias Médicas y Nutrición “Salvador Zubirán” volunteered for the study. Over 50% of participants were from the central area of the country, reflecting the patient population most often seen in our hospital; all had been living in Mexico City for at least the past 6 months.
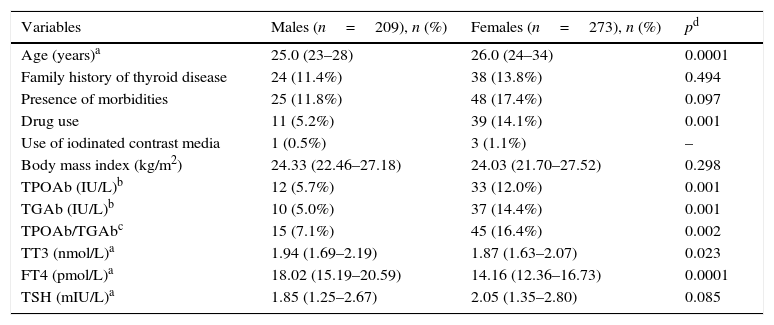
The total population was young, with a median age of 26 and an interquartile range (IQR) between 24 and 30 years; only 11% were above the age of 40, the female/male ratio was 56.5/43.5% and the population's characteristics are described in Table 1.
Characteristics of the total population (n=482).
| Variables | Males (n=209), n (%) | Females (n=273), n (%) | pd |
|---|---|---|---|
| Age (years)a | 25.0 (23–28) | 26.0 (24–34) | 0.0001 |
| Family history of thyroid disease | 24 (11.4%) | 38 (13.8%) | 0.494 |
| Presence of morbidities | 25 (11.8%) | 48 (17.4%) | 0.097 |
| Drug use | 11 (5.2%) | 39 (14.1%) | 0.001 |
| Use of iodinated contrast media | 1 (0.5%) | 3 (1.1%) | – |
| Body mass index (kg/m2) | 24.33 (22.46–27.18) | 24.03 (21.70–27.52) | 0.298 |
| TPOAb (IU/L)b | 12 (5.7%) | 33 (12.0%) | 0.001 |
| TGAb (IU/L)b | 10 (5.0%) | 37 (14.4%) | 0.001 |
| TPOAb/TGAbc | 15 (7.1%) | 45 (16.4%) | 0.002 |
| TT3 (nmol/L)a | 1.94 (1.69–2.19) | 1.87 (1.63–2.07) | 0.023 |
| FT4 (pmol/L)a | 18.02 (15.19–20.59) | 14.16 (12.36–16.73) | 0.0001 |
| TSH (mIU/L)a | 1.85 (1.25–2.67) | 2.05 (1.35–2.80) | 0.085 |
TPOAb: thyroid anti-peroxidase antibodies, TGAb: anti-thyroglobulin antibodies, TT3: total T3, FT4: free T4.
Physical examination and thyroid ultrasound were only performed in 427 volunteers because some of the participants did not continue with the second phase of the evaluation. A flowchart outlines the selection of the reference population from the entire volunteer group (Fig. 1). Several participants had more than one exclusion criterion according to the NACB. The thyroid ultrasound excluded 84 (17.4%) individuals due to structural or echogenicity issues, and the physical examination only excluded 11 (2.4%). US detected one or more thyroid nodules in 9.13% of the studied volunteers vs. 0.7% detected on physical examination; this discrepancy is well known and can be explained by the sensitivity of modern ultrasound equipment. Goiter was found in only 9 volunteers by ultrasound and 8 of them were also detected clinically. Diffuse thyroid hypoechogenicity was a frequent finding, particularly in females; however, grade 3 or marked hypoechogenicity was found in only 6 males (3.3%) and 30 females (12.6%), p=0.0001, who were excluded from the reference population. The median thyroid volume in the total population was 9.32mL with an IQR between 7.07 and 11.79. Six participants were excluded due to thyroid dysfunction: 5 for clinical hypothyroidism (1.04%), 1 for clinical hyperthyroidism (0.20%), and 2 due to subclinical hyperthyroidism (0.41%); these latter had TSH levels <0.1mIU/L and they were mainly excluded because they were the only outliers eliminated by the test for our purposes.
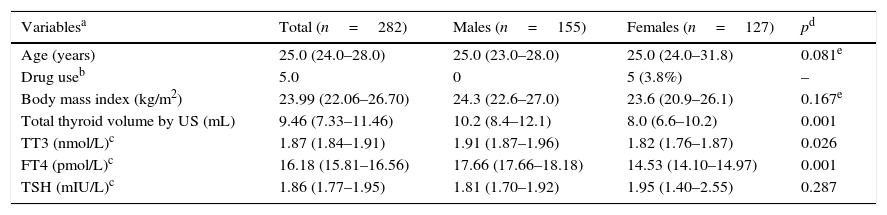
Finally the reference population included 282 volunteers, as 200 participants (41.5%) resulted excluded (see Table 2). As expected, the excluded subjects were mainly females, thus slightly biasing the reference population toward males. The reference group did not differ from the initial population in age or body mass index; only 5 females were on estrogen therapy, but this is an allowed variant that did not require exclusion. Differences in thyroid volume were observed between genders, as expected. In accordance with the Anderson–Darling test, the data distribution of thyroid hormones and antibodies did not follow a normal distribution; however, the TSH, FT4, and TT3 logarithm did have a normal distribution and fulfilled the normality test. The TSH geometric mean value in the reference population was 1.86±1.63mIU/L, the TT3 geometric mean was 1.87±1.20mIU/L, and that of FT4 was 16.18±1.25pmol/L. The difference between genders was statistically significant, except for TSH, which was not different between males and females.
Characteristics of the reference population (n=282).
| Variablesa | Total (n=282) | Males (n=155) | Females (n=127) | pd |
|---|---|---|---|---|
| Age (years) | 25.0 (24.0–28.0) | 25.0 (23.0–28.0) | 25.0 (24.0–31.8) | 0.081e |
| Drug useb | 5.0 | 0 | 5 (3.8%) | – |
| Body mass index (kg/m2) | 23.99 (22.06–26.70) | 24.3 (22.6–27.0) | 23.6 (20.9–26.1) | 0.167e |
| Total thyroid volume by US (mL) | 9.46 (7.33–11.46) | 10.2 (8.4–12.1) | 8.0 (6.6–10.2) | 0.001 |
| TT3 (nmol/L)c | 1.87 (1.84–1.91) | 1.91 (1.87–1.96) | 1.82 (1.76–1.87) | 0.026 |
| FT4 (pmol/L)c | 16.18 (15.81–16.56) | 17.66 (17.66–18.18) | 14.53 (14.10–14.97) | 0.001 |
| TSH (mIU/L)c | 1.86 (1.77–1.95) | 1.81 (1.70–1.92) | 1.95 (1.40–2.55) | 0.287 |
US: ultrasound, TT3: total T3, FT4: free T4.
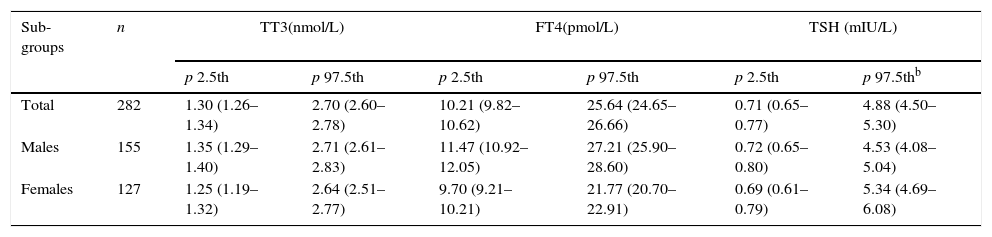
The reference values for TSH, FT4, and TT3 after logarithmic transformation in the reference population are shown in Table 3.
Thyroid hormone values in the reference population (n=282).a
| Sub-groups | n | TT3(nmol/L) | FT4(pmol/L) | TSH (mIU/L) | |||
|---|---|---|---|---|---|---|---|
| p 2.5th | p 97.5th | p 2.5th | p 97.5th | p 2.5th | p 97.5thb | ||
| Total | 282 | 1.30 (1.26–1.34) | 2.70 (2.60–2.78) | 10.21 (9.82–10.62) | 25.64 (24.65–26.66) | 0.71 (0.65–0.77) | 4.88 (4.50–5.30) |
| Males | 155 | 1.35 (1.29–1.40) | 2.71 (2.61–2.83) | 11.47 (10.92–12.05) | 27.21 (25.90–28.60) | 0.72 (0.65–0.80) | 4.53 (4.08–5.04) |
| Females | 127 | 1.25 (1.19–1.32) | 2.64 (2.51–2.77) | 9.70 (9.21–10.21) | 21.77 (20.70–22.91) | 0.69 (0.61–0.79) | 5.34 (4.69–6.08) |
FT4: free T4, TT3: total T3.
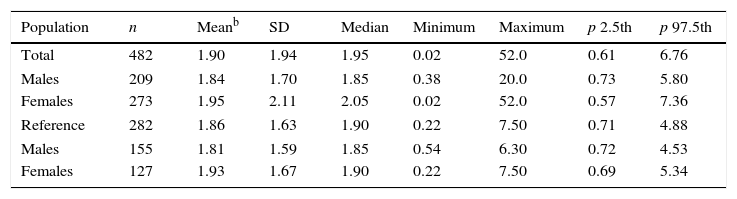
The reference population's median iodiuria was 267μg/L, IQR (161.3–482.5). Table 4 details the TSH reference intervals in both the initial group and the reference population.
Comparison of TSH reference intervals in the total and reference populations.a
| Population | n | Meanb | SD | Median | Minimum | Maximum | p 2.5th | p 97.5th |
|---|---|---|---|---|---|---|---|---|
| Total | 482 | 1.90 | 1.94 | 1.95 | 0.02 | 52.0 | 0.61 | 6.76 |
| Males | 209 | 1.84 | 1.70 | 1.85 | 0.38 | 20.0 | 0.73 | 5.80 |
| Females | 273 | 1.95 | 2.11 | 2.05 | 0.02 | 52.0 | 0.57 | 7.36 |
| Reference | 282 | 1.86 | 1.63 | 1.90 | 0.22 | 7.50 | 0.71 | 4.88 |
| Males | 155 | 1.81 | 1.59 | 1.85 | 0.54 | 6.30 | 0.72 | 4.53 |
| Females | 127 | 1.93 | 1.67 | 1.90 | 0.22 | 7.50 | 0.69 | 5.34 |
TSH: thyrotropin (mIU/L).
This is the first study in the literature reporting the TSH reference intervals in a Mexican population obtained according to the NACB criteria together with normal thyroid US findings. The selection of the reference population was stringent, with exclusion of 41.5% of the initially recruited group. This narrowed the TSH reference interval 0.61–6.76mIU/L in the initial group, to 0.71–4.88mIU/L; this difference probably reflects a substantial number of individuals with pre-clinical thyroid disease in our initial study group.
The TSH upper reference limit established in our reference population was much higher than the value of 2.5mIU/L recommended by the NACB. This finding is in agreement with most epidemiological studies following the NACB criteria,8,9,12 in which this upper limit has also been exceeded. Interestingly, the values obtained in our TSH reference intervals are 1–2 units above those referred to in these studies, and ours is one of the highest for TSH. A possible explanation for this finding may be high iodine dietetic intake, which was confirmed in the individuals in whom we measured iodiuria; the recorded median placed these individuals in a population with a more than adequate iodine nutritional intake (WHO/ICCIDD/UNICEF).13
The most relevant epidemiological studies have been conducted in China, proving the importance of dietary iodine intake. Due to this country's diversity and geographic extension, they were able to follow populations with different dietary iodine content, ranging from deficient to excessive. Their results have been most enlightening; the cohort exposed to high rich intake had an increased prevalence and incidence of autoimmune thyroiditis and, hence, clinical and subclinical hypothyroidism.14–16 A five-year follow-up of this cohort also showed a greater cumulative incidence of anti-thyroglobulin antibodies.17 The highest TSH reference interval (0.59–5.98mIU/L) was found in the regions with excessive iodine intake when compared to those with deficient or more than adequate dietary iodine.18,19 Our reference interval is similar to that reported in their population with the highest iodine intake.
Dietary iodine is one of the main recognized environmental factors that influence thyroid function. The relation between iodine intake and the risk of developing thyroid disease follows a U pattern, with increased risk for both minimal and excessive intake.20 Excessive iodine intake and its repercussions have been broadly studied in the past few decades from a pathophysiological and epidemiological viewpoint. Though the normal thyroid gland is able to adapt to excess iodine, this adjustment is not perfect, and T4 and TSH levels suggest disequilibrium in glandular secretion. The main adaptive mechanism to iodine excess hinges on downregulation of the Na+/I− symporter (NIS), with decreased transport gradient into the thyrocyte.21
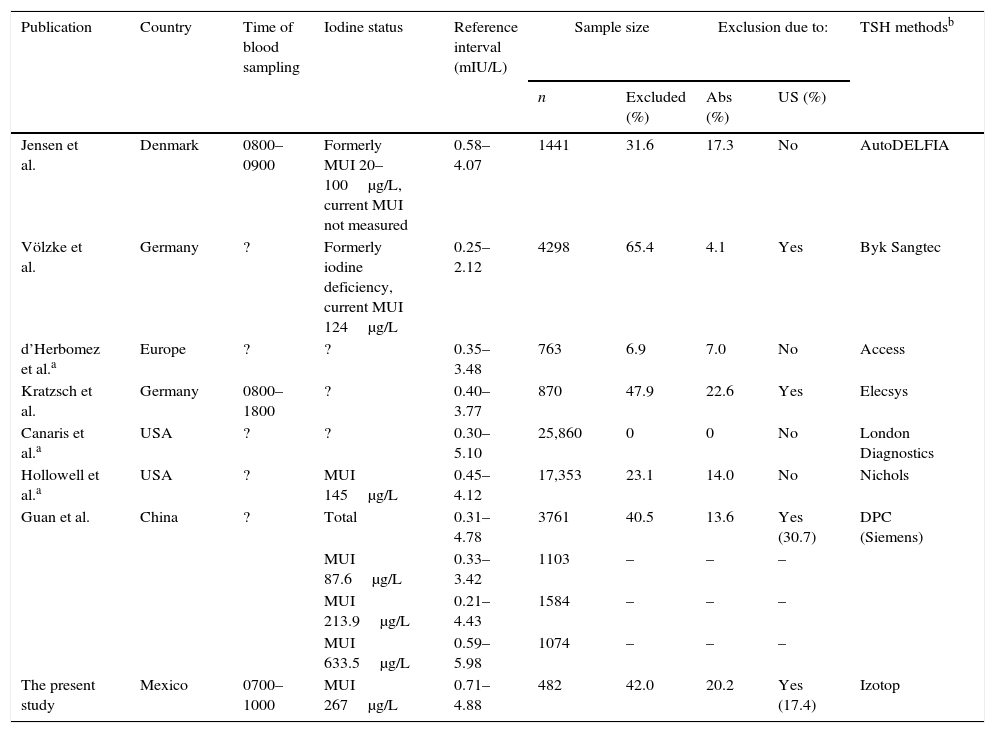
Iodine has been claimed to induce or trigger thyroid autoimmunity on the basis of animal studies suggesting a strong link between iodine intake and the development of autoimmune thyroiditis. In particular, excessive iodine intake in genetically susceptible animals accelerates thyroiditis development, whereas low iodine intake attenuates this effect.20 There are several observational studies in different populations ranging from low to sufficient, and even excessive dietary iodine, as well as controlled interventional studies in which iodinated oils or potassium iodide were supplemented to iodine-deficient populations. These studies confirmed an increased prevalence of anti-thyroid antibodies and autoimmune thyroiditis.22–25 However, factors other than iodine intake may explain different results among studies based on the NACB criteria. These have been pertinently pointed out by Jensen et al.26 and include the time period since sample collection, the equipment, and the type of assay used (Table 5). TSH is known to have various glycoforms, differently recognized by different immunoassays, thus contributing to the difficult method standardization and leading to discordant results even in the serum of one individual; these differences may even account for a 30–40% variation in TSH result, exclusively attributable to the type of assay technology.27
Reference intervals of TSH obtained from recent studies.
| Publication | Country | Time of blood sampling | Iodine status | Reference interval (mIU/L) | Sample size | Exclusion due to: | TSH methodsb | ||
|---|---|---|---|---|---|---|---|---|---|
| n | Excluded (%) | Abs (%) | US (%) | ||||||
| Jensen et al. | Denmark | 0800–0900 | Formerly MUI 20–100μg/L, current MUI not measured | 0.58–4.07 | 1441 | 31.6 | 17.3 | No | AutoDELFIA |
| Völzke et al. | Germany | ? | Formerly iodine deficiency, current MUI 124μg/L | 0.25–2.12 | 4298 | 65.4 | 4.1 | Yes | Byk Sangtec |
| d’Herbomez et al.a | Europe | ? | ? | 0.35–3.48 | 763 | 6.9 | 7.0 | No | Access |
| Kratzsch et al. | Germany | 0800–1800 | ? | 0.40–3.77 | 870 | 47.9 | 22.6 | Yes | Elecsys |
| Canaris et al.a | USA | ? | ? | 0.30–5.10 | 25,860 | 0 | 0 | No | London Diagnostics |
| Hollowell et al.a | USA | ? | MUI 145μg/L | 0.45–4.12 | 17,353 | 23.1 | 14.0 | No | Nichols |
| Guan et al. | China | ? | Total | 0.31–4.78 | 3761 | 40.5 | 13.6 | Yes (30.7) | DPC (Siemens) |
| MUI 87.6μg/L | 0.33–3.42 | 1103 | – | – | – | ||||
| MUI 213.9μg/L | 0.21–4.43 | 1584 | – | – | – | ||||
| MUI 633.5μg/L | 0.59–5.98 | 1074 | – | – | – | ||||
| The present study | Mexico | 0700–1000 | MUI 267μg/L | 0.71–4.88 | 482 | 42.0 | 20.2 | Yes (17.4) | Izotop |
Modified from Ref. 26 (reproduced with permission from the American Association for Clinical Chemistry).
MUI: median urinary iodine, Abs: antibody, US: ultrasound.
Another finding in our study is the significant difference between our FT4 and TT3 results and reference values; we observed higher levels in males than in females, a fact that is disclosed neither in the commercial kits nor in the literature, although it has been mentioned for FT4 in some reports.28
As for the study limitations, they primarily lie on the included population, a small group mainly composed of young adults (with a median age of 26). However, according to the American Thyroid Association (ATA) guidelines, the age range of this population includes the recommended age for the start of screening for thyroid dysfunction in asymptomatic adults.29
The equipment used for hormone concentration measurements is other limitation, because we used isotopic immunoassays. However, although these are presently less commonly used throughout the world, they are still routine methods in Mexico, particularly in large public health institutions.
The determination of urinary iodine concentrations in samples obtained exclusively from our reference population is another limitation. The number of individuals in whom urinary iodide was determined is that recommended for establishing median iodide excretion in a group; moreover, this median coincides with dietary iodine intake determined by WHO/ICCIDD for our country in the most recent studies conducted in Mexico (235μg/L).30
Our study's pivotal strength is the strict rigor followed in the selection of the reference population, with elimination of a large subset of participants with potential subclinical disease, whose inclusion could have increased the TSH upper limit values and biased the final results.
Aside from its fully recognized limitations, this is the only study conducted in Latin America with such a rigorous population selection, and although its characteristics may preclude the extrapolation of results to other populations, it is a reference point in our country and the surrounding areas.
In conclusion, this is the first study reporting the TSH reference interval in a Mexican population, following the NACB criteria and correlating them with thyroid ultrasound normality. The obtained interval is 0.71–4.88mIU/L, with a median ioduria of 267μg/L in this same population.
Conflict of interestThe authors have no conflict of interest or financial or personal relationship to the study and declare receiving no funding or grant support.
We gratefully acknowledge Ms. Carolina Martínez from the Nutritional Biochemistry Laboratory of the Instituto de Nutrición de CentroAmérica y Panamá (INCAP), the IRLI laboratory for urinary iodine determinations and the members of the Latin American Thyroid Society that generously provided valuable comments on the study, particularly, Dr. Liliana Bergoglio (Argentina) and Dr. Ofelia González-Treviño (Mexico).