Universal thyroid function screening in pregnant women is one of the most controversial issues in current endocrinology. In contrast to the American Thyroid Association (ATA) clinical guidelines, which recommend selective screening in the population at risk,1 other scientific societies, including the Spanish Society of Endocrinology and Nutrition (SEEN), advocate universal screening in the pregnant population.2 However, reference ranges for thyroid-stimulating hormone (TSH) and free thyroxine (FT4) levels in pregnant women proposed by the ATA are assumed without the necessary critical assessment. An article published by Lombardo Grifol et al. emphasizes this, as had already been done by the prior studies published in the dissemination organ of SEEN.3
It is particularly significant that TSH reference ranges in Spanish populations from very distant geographical areas, obtained using different laboratory procedures and statistical methods, greatly differ from those recommended by international guidelines (TSH<2.5μUI/ml during the first trimester of pregnancy) and are very similar to each other.3–8
On January 2013, the Hospital Clínico Universitario in Valladolid started universal thyroid function screening in pregnancy in collaboration with the departments of gynecology and obstetrics and clinical laboratory. During 2013, TSH and FT4 levels and thyroid autoimmunity were retrospectively tested in 1.316 women (mean age: 32.6±5.6 years) in the week 10 of pregnancy (Cobas® 6000, Roche Diagnostics). One hundred and sixty women were excluded for positive autoimmunity (115 women, 8.7%), prior thyroid disease and/or treatment modifying thyroid profile. Reference ranges for TSH and FT4 in week 10 of pregnancy were calculated according to recommendations by the Internacional Federation of Clinical Chemistry (IFCC). For this, distribution of FT4 and TSH levels was normalized by logarithmic transformation, and confidence intervals for the 2.5th and 97.5th percentiles, corresponding to the lower and upper limits of the reference values respectively, were subsequently calculated.9
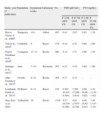
The results recorded in our population are similar to those previously reported for the Spanish population, with minimal differences attributable to the gestational week, the procedure used, and the area of origin (Table 1),3–8 but significantly differ from those recommended by the ATA and SEEN.1,2 This striking situation is not unique, because recent studies reported similar reference ranges in healthy pregnant women from other countries. This stresses the importance of calculating reference ranges for each laboratory.10 In this regard, it should be noted that American and Spanish guidelines advise TSH levels under 2.5μUI/ml only if no reference values are available for procedures used and for the same study population during the first trimester. However, in light of the results, hormone levels in Spanish populations are consistently higher regardless of the procedure used and the population tested. This should be reflected in the future in the clinical guidelines/recommendations published by the SEEN in this regard. It should not be forgotten that no adequate scientific evidence is available on the efficacy of treatment for subclinical hypothyroidism with TSH levels higher than 2.5μUI/ml in pregnant women.11,12
Normal TSH and FT4 levels in the Spanish population with negative autoimmunity.
| Study, year of publication | Population | Gestational weeks | Laboratory | No. | TSH (μIU/mL) | FT4 (ng/dL) | ||
| P 2.5th (90% CI) | P 97.5th (90% CI) | P 2.5th (90% CI) | P 97.5th (90% CI) | |||||
| Bocos Terraz et al., 20094 | Saragossa | <14 | Abbot | 481 | 0.41 | 2.63 | 0.83 | 1.38 |
| Vila et al., 20105 | Catalonia | 9 | Bayer | 178 | 0.12 | 4.75 | 0.80 | 1.60 |
| García Guadiana et al., 20106 | Cartagena | 11–13 | Roche | 400 | 0.13 | 3.71 | 0.89 | 1.50 |
| Santiago et al., 20117 | Jaén | 7–10 | Beckman | 305 | 0.23 | 4.18 | 0.60 | 1.06 |
| Aller Granda et al., 20138 | Oviedo | 6–12 | Roche | 264 | 0.17 | 4.15 | – | – |
| Lombardo Grifol et al., 20133 | El Bierzo | 8–13 | Bayer | 219 | 0.497 (0.415–0.584) | 3.595 (3.298–3.914) | 0.90 (0.88–0.92) | 1.42 (1.39–1.45) |
| Díaz-Soto et al., 2014 | Valladolid | 10 | Roche | 1156 | 0.27 (0.159–0.346) | 4.05 (3.973–4.170) | 0.94 (0.92–0.95) | 1.50 (1.47–1.55) |
Data from this study are given in italics.
CI: confidence interval; P: percentile; TSH: thyroid-stimulating hormone; FT4: free thyroxine.
Setting a given reference range obviously conditions clinical practice, but also has important economic and care implications. During 2013, an upper TSH limit of 2.5μUI/ml would have implied monitoring and treatment with levothyroxine of 436 pregnant women of our population, i.e. 38%, while 130 women (11%) would have been treated based on the normal criteria calculated for our area (TSH≥4.05μUI/ml).
It should also be stressed that only 62 (5%) of all women diagnosed with primary hypothyroidism during 2013 had TSH levels≥5μUI/ml, and of these, only 7 pregnant women (0.6%) had TSH levels≥10μUI/ml which could be classified as having frank hypothyroidism.
In conclusion, we think that universal screening for thyroid dysfunction is warranted, because it allows for adequate diagnosis and management of a small but significant proportion of pregnant women with hypothyroidism which could affect the adequate course of pregnancy and, probably, fetal and infant development.11,12 Universalization of screening requires, however, calculation of reference values for the population and the laboratory procedure of each hospital in a given gestational week.6 This analysis should not be considered as an exceptional method in the setting of research studies, but as a test of maximal interest for care, and is mandatory before any system for screening gestational thyroid dysfunction is implemented. Setting a universal cut-off point without considering the characteristics of each population (iodine intake, subclinical autoimmune disease, etc.) not only implies a high care overload with its attendant financial expense, but also a significant psychological burden during a especially sensitive period, as well as overtreatment of a great proportion of the population with the resultant additional risk.
FundingThis study received no public or private funding.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Díaz-Soto G, Largo E, Álvarez-Colomo C, Martínez-Pino I, de Luis D. Valores de referencia y cribado universal de la disfunción tiroidea en la mujer gestante. Endocrinol Nutr. 2014;61:336‐338.