Struma ovarii is an uncommon teratoma of the ovary that contains over 50% of thyroid tissue. This tumor accounts for 0.2-1.3% of all ovarian tumors and for 2-4% of all teratomas. Malignancy is reported in 5% to 37% of patients with struma ovarii, with metastases occurring in few cases.1–3 This article reports a patient with some unusual features, including malignancy, metastases, hormone production, and a fatal outcome. Literature is reviewed, and significance of appropriate treatment and follow-up of these patients is emphasized.
A 38-year-old woman was referred to our hospital in 1994 for a second opinion regarding management of a struma ovarii. The disease had been diagnosed at another center in 1981, when she was 25 years old, after right salpingectomy and partial oophorectomy for a right ovarian cyst. At that time, pathological study showed a struma ovarii with no signs of malignancy. In 1993 (at the age of 37 years), patient underwent a left salpingo-oophorectomy and completion of right oophorectomy because of detection of an 8-cm left ovarian mass. Multiple peritoneal nodules were found during surgery, and pathological study found a struma ovarii with positive immunohistochemistry for thyroglobulin. A 131I scan revealed physiological accumulation of iodine in the thyroid gland and pathological deposits in abdomen. Total thyroidectomy and 131I ablation therapy were proposed, but the patient refused them.
When patient attended our center in 1994, abdominal computed tomography (CT) showed nodules throughout the peritoneal cavity, and a 123I scan revealed high uptake in the right iliac bone. A bone biopsy identified metastasis from a follicular carcinoma, reporting malignancy for the first time. The patient again refused thyroidectomy and 131I ablation therapy, but continued to return for follow-up visits.
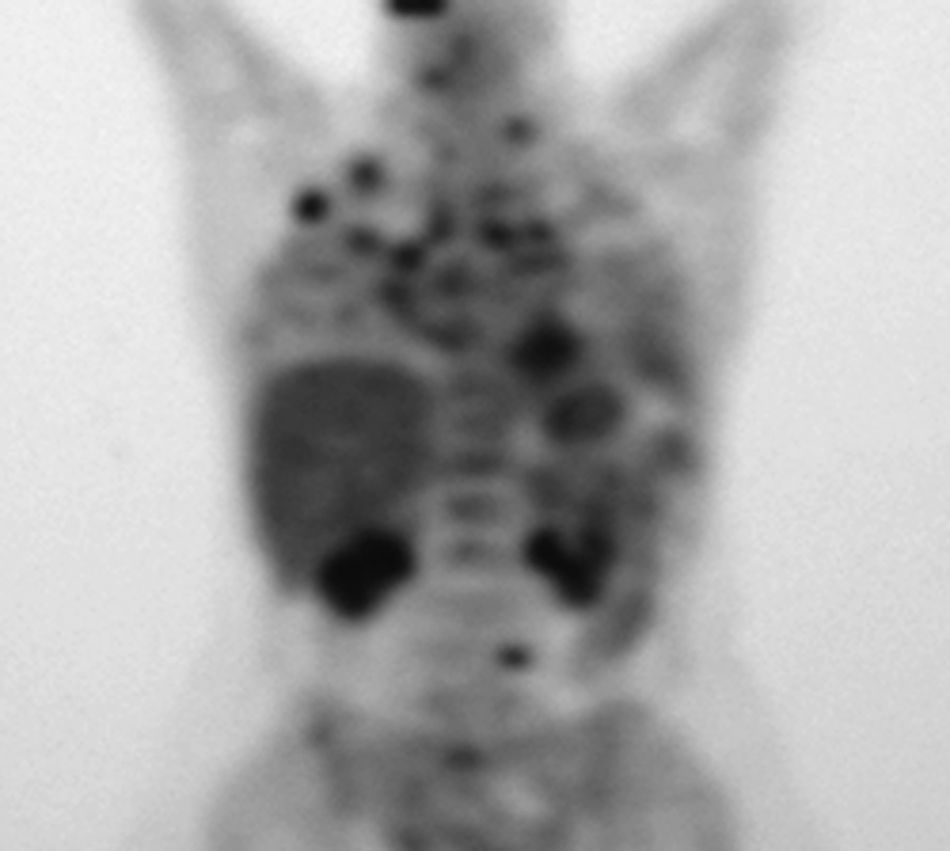
Between 1994 and September 2008, thyroglobulin levels varied from 600 to 1,600 mcg/L (normal <60 mcg/L) and peritoneal lesions increased in number and size. In September 2008, a whole body positron emission tomography showed lung extension, with several nodules in the right lobe and invasion of thoracic lymph nodes (fig. 1). A transbronchial biopsy was consistent with a poorly differentiated carcinoma and immunohistochemistry was non-conclusive. The patient finally agreed to total thyroidectomy, and the pathology laboratory reported a hyperplastic thyroid gland.
A whole body positron emission tomography performed in 2008 revealed a pulmonary hypermetabolic nodule 25mm in diameter in the right upper lobe; several smaller nodules in the right lower lobe; invasion of the left supraclavicular, mediastinal, hilar, and subcarinal lymph nodes; a paraaortic retroperitoneal mass; and multiple focal bone lesions.
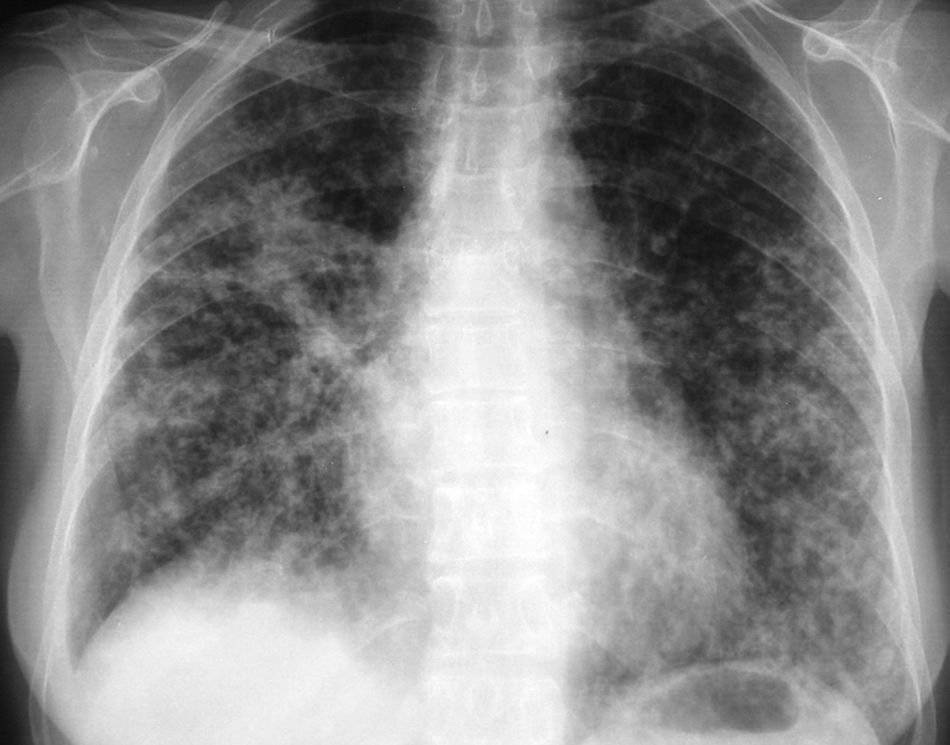
In November 2008, she was admitted for right transverse sinus thrombosis and multiple cerebral infarction from which she recovered completely; no etiology was found. One month later, the patient was readmitted to our center with dyspnea and no symptoms of infection. Chest X-rays showed a bilateral micronodular pattern (fig. 2), serum thyroglobulin level was 6,393 mcg/L, and TSH level was 3.15 mIU/L without thyroid replacement therapy. As the rapid spread of the disease in the lungs was considered the most probable cause of her current condition and thyroidectomy had already been performed, she was treated with 100 mCi of 131I. Twenty-four hours later, patient experienced left hemiparesia, dysarthria, anosognosia and hemianopsia. A brain CT ruled out hematoma, and a second cerebral ischemic event was diagnosed. Over the following days, patient experienced respiratory insufficiency and worsening of X-ray signs, and finally died.
Struma ovarii is typically diagnosed before menopause. Benign lesions usually occur in the 4th decade of life, and malignant tumors in the 5th decade. The disease generally occurs as a unilateral mass, but up to 6% of cases are bilateral.2,4 Most patients are asymptomatic or have nonspecific signs or symptoms such as pelvic pain, menstrual cycle changes, or an abdominal mass.3–5 Like other ovarian tumors, struma ovarii may also cause ascites and hydrothorax, an indolent condition called pseudo-Meigs syndrome.3,6 Hyperthyroidism appears in 5-8% of struma ovarii patients and is more frequent in benign lesions; however, when hyperthyroidism occurs in a malignant lesion, metastases are present in up to 83% of cases and are usually large.2,7,8
Diagnostic imaging techniques such as ultrasonography, CT, or magnetic resonance imaging with gadolinium show a heterogeneous ovarian tumor. Pathological study is the only way to confirm diagnosis of struma ovarii. Gross appearance is a large, green-brown, solid-cystic tumor with other features typical of teratomas.1 Microscopically, hyperplastic foci, thyroiditis, adenoma, or carcinoma may be seen.2,8 Immunohistochemical staining for thyroglobulin, chromogranin, and more recently with TTF-1 (thyroid transcription factor-1) is crucial for diagnosis of struma ovarii and helps differentiate struma from carcinoid, ovarian adenocarcinoma, and granulosa cell tumors.1,2
Malignancy criteria are controversial. Geist and Smith initially defined malignancy as struma ovarii presenting with cellular atypia, vascular invasion, and metastases.5 The tumor may spread through regional lymph nodes, hematological dissemination to bone, lung, liver and brain, and directly to the omentum, peritoneal cavity, or contralateral ovary.1,5 However, since peritoneal strumosis has an indolent clinical course, as in the reported case, some authors advocate that metastases can no longer be considered a sign of malignancy.3,9 For this reason, to define malignancy Devaney et al1 only consider cytological findings such as vascular invasion, intense mitotic activity, or overlapping “ground glass” nuclei.
Therapeutic approach to these tumors is also controversial. Treatment of benign struma ovarii is unilateral oophorectomy, but treatment of malignant tumors is more complex. Hysterectomy and salpingo-oophorectomy with omentectomy and peritoneal lymphadenectomy must be performed. Total thyroidectomy is then performed for monitoring thyroglobulin levels and performance of 131I scans during follow-up. After thyroidectomy, suppressive levothyroxine treatment must be given, but 131I ablation therapy is still controversial. While some authors recommend this in all patients,5 others reserve it for cases with locoregional extension, metastases, or recurrent disease.1,6 An exception to this complex treatment would be fertile women with malignant localized tumor (stage Ia), in whom unilateral oophorectomy is indicated in consideration of future conception.1,2 Finally, when functional metastases are present, treatment is challenging. As occurred in this case, after oophorectomy and thyroidectomy metastases may produce adequate amounts of thyroid hormone to maintain TSH levels near or even below normal.10 In such cases, 131I ablation therapy may be suboptimal because of the low iodine uptake by the residual tumor, and the optimal way to achieve a cytotoxic concentration of 131I under these circumstances has not been defined. Approaches include a high dose of 131I and 131I with recombinant human TSH.7,8
In the case reported, patient developed no complications for 27 years despite the presence of a malignant lesion with metastases and the lack of adequate treatment. This confirms the very good prognosis and high survival rates of patients with struma ovarii, even when malignancy is confirmed. However, these tumors may be life-threatening if left untreated, as occurred in our case. Monitoring with thyroglobulin measurements, 131I scans, and positron emission tomography in some selected patients is therefore essential to plan adequate treatment for patients with this complex condition.