The triglyceride/HDL cholesterol ratio, as a surrogate marker of insulin resistance, may be associated to presence of subclinical carotid atherosclerosis in postmenopausal women. The aim of this study was to explore this association.
Patients and methodsWomen (last menstrual period≥2 years) in primary prevention up to 65 years of age were recruited. Association between the triglyceride/HDL cholesterol (HDL-C) ratio and presence of carotid plaque, assessed by ultrasonography, was analyzed. ROC analysis was performed, determining the precision of this ratio to detect carotid plaque.
ResultsA total of 332 women (age 57±5 years) were recruited. Triglyceride/HDL-C ratio was 2.35±1.6. Prevalence of carotid plaque was 29%. Women with carotid plaque had higher triglyceride/HDL-C ratios (3.33±1.96 vs. 2.1±1.2, p<0.001) than women with no carotid plaque. A positive relationship was seen between quintiles of this ratio and prevalence of carotid plaque (p<0.001). Regardless of other risk factors, women with higher triglyceride/HDL-C ratios were more likely to have carotid plaque (odds ratio 1.47, 95% confidence interval 1.20–1.79, p<0.001). The area under the curve of the triglyceride/HDL-C ratio to detect carotid plaque was 0.71 (95% confidence interval 0.65 to 0.76), and the optimal cut-off point was 2.04.
ConclusionsIn postmenopausal women in primary prevention, insulin resistance, estimated from the triglyceride/HDL-C ratio, was independently associated to a greater probability of carotid plaque. A value of such ratio greater than 2 may be used for assessing cardiovascular risk in this particular group of women.
La relación triglicéridos/colesterol HDL, como marcador subrogado de insulinor resistencia, podría asociarse a la presencia de ateromatosis subclínica carotídea en mujeres posmenopáusicas. El objetivo de nuestro trabajo fue explorar dicha asociación.
Pacientes y métodosSe incluyeron mujeres (última menstruación≥2 años) en prevención primaria de una edad hasta 65 años. Se analizó la asociación entre la relación triglicéridos/colesterol HDL (C-HDL) y la presencia de placa carotídea cuantificada por ecografía. Se realizó un análisis ROC, calculando la precisión de dicha razón para detectar placa carotídea.
ResultadosSe incluyeron 332 mujeres (edad 57±5 años). La razón triglicéridos/C-HDL fue 2,35±1,6. La prevalencia de placa carotídea fue 29%. Las mujeres con placa carotídea mostraron una mayor razón triglicéridos/C-HDL (3,33±2,1 vs. 1,96±1,2, p<0,001) en comparación con las mujeres sin placa. Se observó una relación positiva entre los quintilos de dicha razón lipídica y la prevalencia de placa carotídea (p<0,001). Independientemente de otros factores de riesgo, las mujeres con una razón triglicéridos/C-HDL más elevada mostraron mayor probabilidad de presentar placa carotídea (odds ratio 1,47, intervalo de confianza 95%: 1,20-1,79, p<0,001). El área bajo la curva de la razón triglicéridos/C-HDL para detectar placa carotídea fue 0,71 (intervalo de confianza 95%: 0,65-0,76) y el punto de corte óptimo 2,04.
ConclusionesEn mujeres posmenopáusicas en prevención primaria la insulinor resistencia estimada a partir de la razón triglicéridos/C-HDL se asoció independientemente con una mayor probabilidad de presentar placa carotídea. El valor de dicha relación mayor a 2 podría utilizarse en la evaluación del riesgo cardiovascular en este grupo particular de mujeres.
Cardiovascular disease occurs less commonly in premenopausal women as compared to men of similar ages.1 After menopause this difference disappears, and the situation may even be inverted at more extreme ages.2 Traditionally, the loss of estrogen protection was the main mechanism proposed to explain such findings.3 There are however other factors, such as the high prevalence in menopause of metabolic syndrome (and its pathophysiological substrate, insulin resistance), which could also account for the increased incidence of cardiovascular events after childbearing age.4–7
Insulin resistance (IR) is characterized by a decreased biological function of insulin. High plasma insulin levels are required to maintain the metabolism of carbohydrates, protein, and lipids. The Homeostasis Model Assessment (HOMA) is routinely used to estimate IR and beta cell function based on fasting plasma glucose and insulin levels.8 Other markers for estimating or predicting IR have also been assessed. These include the triglycerides/HDL cholesterol (TG/HDL-C) ratio, considered a surrogate parameter of IR.9–12
The incorporation of carotid intima-media thickness and, in particular, the presence of carotid atherosclerotic plaque (CAP) in a model consisting of the traditional risk factors improves cardiovascular risk prediction in both men and women.13 Our work group showed a substantial prevalence of CAP in middle-aged postmenopausal women, despite the fact that they were classified as having low cardiovascular risk according to different risk equations.14
Prior studies showed that greater intima-media thickness was associated with higher TG/HDL ratios in children, adolescents, and adults.15,16 The association of that marker of IR with the presence of CAP in middle-aged postmenopausal women in our region is unknown.
Based on the above considerations, this study was intended to: (1) estimate the TG/HDL ratio in a population of middle-aged postmenopausal women in primary prevention; (2) ascertain the association between the TG/HDL ratio and the presence of CAP; and (3) estimate the accuracy of the TG/HDL ratio for detecting CAP, establishing the optimal cut-off point that discriminates between women with or without evidence of CAP.
Patients and methodsA multicenter, descriptive, cross-sectional study was conducted of consecutive samples taken at six cardiovascular prevention clinics in Buenos Aires, Argentina.
The inclusion criteria were: women aged≤65 years with ≥2 years since their last menstrual period.
The exclusion criteria were: prior cardiovascular disease (acute myocardial infarction, unstable angina, stable chronic angina, myocardial revascularization surgery, coronary angioplasty, stroke, peripheral vascular disease, disease in the aorta or any of its branches); a history of diabetes mellitus; prior lipid-lowering treatment and hormone replacement therapy.
The TG/HDL ratio was obtained by dividing the triglyceride level (mg/dL) by the HDL-C level (mg/dL). The 10-year Framingham score for fatal or non-fatal coronary events used by the third report of the expert panel of the National Cholesterol Education Program on the detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) was calculated. The population was defined as being at low, moderate, or high risk if the risk was <10%, 10–19%, and ≥20% respectively.17
CAP was defined as the noninvasive detection of atherosclerotic plaque in the carotid arteries by two-dimensional ultrasonography using a linear transducer. The presence of plaque was defined based on the following criteria: (1) abnormal wall thickness (intima-media thickness>1.5mm); (2) abnormal structure; and (3) abnormal wall echogenicity.
The association of the TG/HDL ratio and the presence of CAP was determined using univariate and multivariate analysis (adjusted for age, systolic blood pressure, body mass index, smoking, and total cholesterol). The strength of the association was expressed as an odds ratio and its respective 95% confidence interval (95% CI).
A receiver operating characteristic (ROC) curve analysis was performed, determining the area under the curve to accurately assess the TG/HDL ratio for discriminating between women with or without CAP. The Youden index, corresponding to the maximum vertical distance between the ROC curve and the statistical chance line (CJ point), was used to determine the optimal cut-off point of the TG/HDL ratio for detecting CAP. The sensitivity and specificity of the cut-off point were calculated. Continuous data between two groups were analyzed using a Student's t test if the variables were normally distributed or with a Wilcoxon–Mann–Whitney test otherwise. Categorical data analysis was performed using a Chi-squared test. Continuous variables are given as mean±standard deviation, while categorical variables are given as percentages. A value of p<0.05 was considered statistically significant.
The study was conducted in compliance with the recommendations for medical research contained in the Declaration of Helsinki, Good Clinical Practice standards, and the applicable ethical regulations.
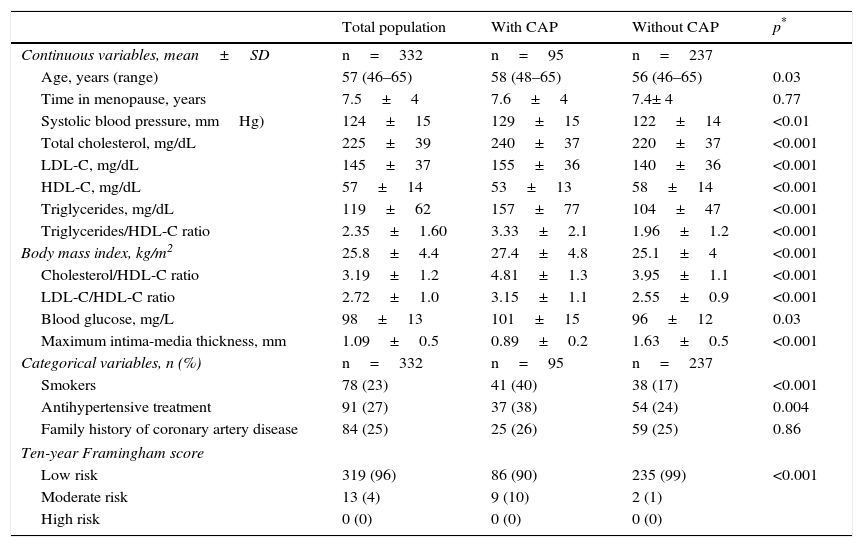
ResultsA total of 332 females with a mean age of 57±years were recruited. Mean plasma triglyceride and HDL-C levels were 119±62mg/dL and 57±14mg/dL respectively. The mean TG/HDL ratio was 2.35±1.6. According to the 10-year Framingham score, 96% of the population was at low risk, and only 4% was categorized as having a moderate risk. There were no women with high cardiovascular risk. Table 1 shows the characteristics of the population.
Baseline characteristics of the population.
| Total population | With CAP | Without CAP | p* | |
|---|---|---|---|---|
| Continuous variables, mean±SD | n=332 | n=95 | n=237 | |
| Age, years (range) | 57 (46–65) | 58 (48–65) | 56 (46–65) | 0.03 |
| Time in menopause, years | 7.5±4 | 7.6±4 | 7.4± 4 | 0.77 |
| Systolic blood pressure, mmHg) | 124±15 | 129±15 | 122±14 | <0.01 |
| Total cholesterol, mg/dL | 225±39 | 240±37 | 220±37 | <0.001 |
| LDL-C, mg/dL | 145±37 | 155±36 | 140±36 | <0.001 |
| HDL-C, mg/dL | 57±14 | 53±13 | 58±14 | <0.001 |
| Triglycerides, mg/dL | 119±62 | 157±77 | 104±47 | <0.001 |
| Triglycerides/HDL-C ratio | 2.35±1.60 | 3.33±2.1 | 1.96±1.2 | <0.001 |
| Body mass index, kg/m2 | 25.8±4.4 | 27.4±4.8 | 25.1±4 | <0.001 |
| Cholesterol/HDL-C ratio | 3.19±1.2 | 4.81±1.3 | 3.95±1.1 | <0.001 |
| LDL-C/HDL-C ratio | 2.72±1.0 | 3.15±1.1 | 2.55±0.9 | <0.001 |
| Blood glucose, mg/L | 98±13 | 101±15 | 96±12 | 0.03 |
| Maximum intima-media thickness, mm | 1.09±0.5 | 0.89±0.2 | 1.63±0.5 | <0.001 |
| Categorical variables, n (%) | n=332 | n=95 | n=237 | |
| Smokers | 78 (23) | 41 (40) | 38 (17) | <0.001 |
| Antihypertensive treatment | 91 (27) | 37 (38) | 54 (24) | 0.004 |
| Family history of coronary artery disease | 84 (25) | 25 (26) | 59 (25) | 0.86 |
| Ten-year Framingham score | ||||
| Low risk | 319 (96) | 86 (90) | 235 (99) | <0.001 |
| Moderate risk | 13 (4) | 9 (10) | 2 (1) | |
| High risk | 0 (0) | 0 (0) | 0 (0) | |
SD: standard deviation; CAP: carotid atherosclerotic plaque.
The prevalence of CAP in the population was 29%. Women with CAP showed significantly greater TG/HDL ratios (3.33±2.1 vs. 1.96±1.2, p<0.001) as compared to women with no CAP. Patients with PAC were also older (58±5 years vs. 56±5 years, p=0.03) and had a greater body mass index (27±5 vs. 25±4, p<0.001), higher systolic blood pressure (129±15mmHg vs. 122±14mmHg, p<0.01) and a greater prevalence of smoking (40% vs. 17%, p<0.001) as compared to women without CAP. Table 1 shows the characteristics of the groups with and without CAP.
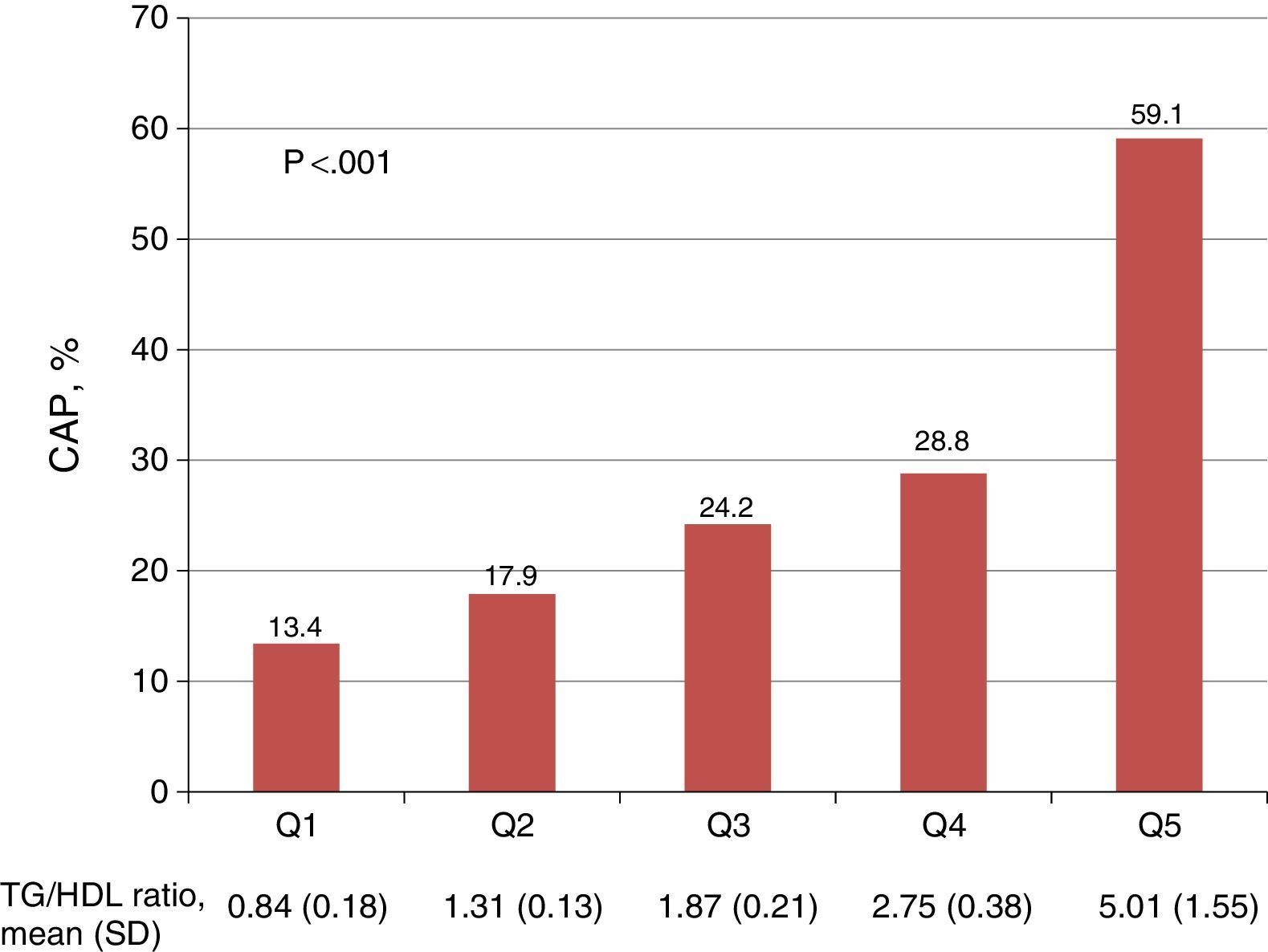
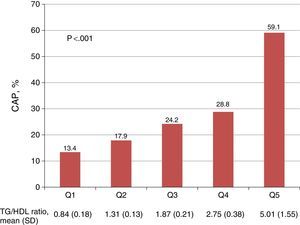
A positive relationship was seen between quintiles of the TG/HDL ratio and the prevalence of CAP (Fig. 1). Women in the lowest quintile of the TG/HDL ratio had a 13.4% prevalence of CAP, as compared to the 59.1% prevalence found in patients in the highest quintile (p<0.001).
Association between quintiles of the triglycerides/HDL cholesterol ratio and the presence of carotid atherosclerotic plaque. The p value corresponds to the comparison between the 1st and 5th quintiles of the TG/HDL ratio. CAP: carotid atherosclerotic plaque; TG/HDL ratio: triglycerides/HDL-C ratio; SD: standard deviation; Q: quintile.
In the multivariate analysis, irrespective of age, systolic blood pressure, total cholesterol, the prevalence of smoking, and the body mass index, women with higher TG/HDL ratios (by each additional unit of the ratio) had a greater chance of having CAP (odds ratio 1.47, 95% CI: 1.20–1.79, p<0.001).
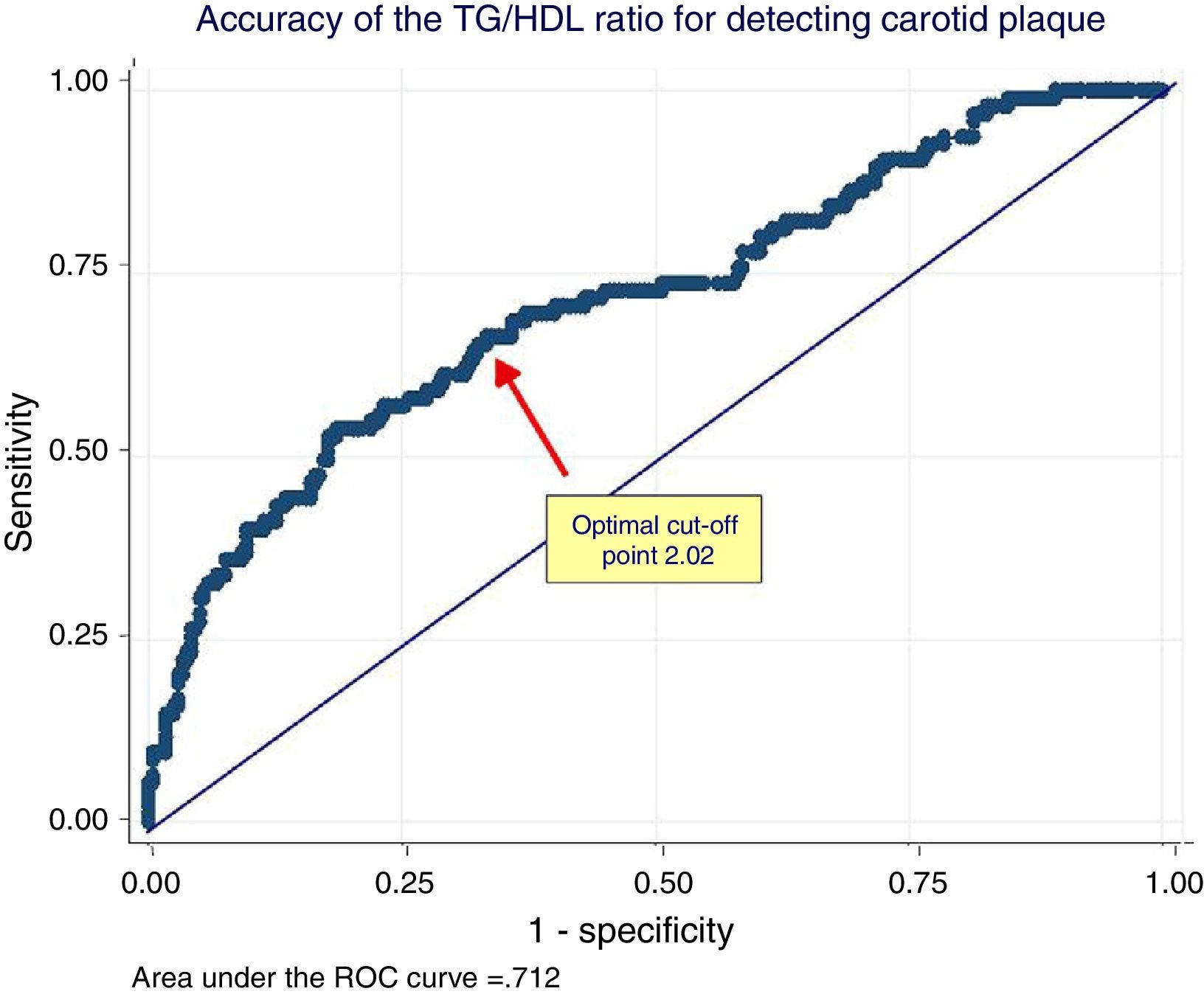
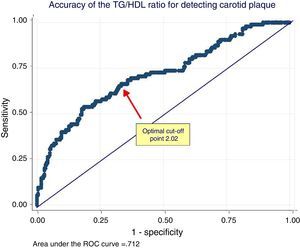
In the ROC analysis, the area under the curve of the TG/HDL ratio for detecting CAP was 0.71 (95% CI: 0.65–0.76) and the optimal cut-off point 2.04 (Youden 0.32). This cut-off value had 70% sensitivity and 63% specificity. The ROC curve is shown in Fig. 2.
ROC analysis of the relationship between the triglycerides/HDL cholesterol ratio and the presence of carotid plaque. The arrow shows the optimal cut-off point of the TG/HDL ratio for discrimination between women with or without carotid plaque. TG/HDL ratio: triglycerides/HDL cholesterol ratio.
Our study showed that in this group of middle-aged, non-diabetic postmenopausal women, IR estimated from the TG/HDL ratio was independently associated with a greater chance of CAP.
The relationship between postmenopausal status and carotid thickening and/or the presence of CAP has been reported previously.18 More importantly, Kablak et al. showed that carotid intima-media thickening predicts for cardiovascular events in pre- and postmenopausal women.19
The greater occurrence of subclinical atheromatosis in postmenopausal women may be explained, at least partly, by the significant physiological changes occurring in women during this stage of their lives. With the disappearance of follicles and estrogens, the postmenopausal ovary mainly secretes androstenedione and testosterone. This results in dramatic changes in the androgen/estrogen ratio after menopause.20 Many such changes imply modifications in some traditional cardiovascular risk factors such as dyslipidemia, body fat amount or distribution, or the appearance of IR.7
For example, a study conducted in Brazil evaluating postmenopausal women found that the presence of subclinical atheromatosis was associated with a greater prevalence of metabolic syndrome and C-reactive protein.21 Levels of triglycerides and HDL-C, lipid markers related to metabolic syndrome and IR, have been independently associated with a greater prevalence of CAP.22 Although the ratio between those lipid markers is a marker of IR, changes in triglyceride and HDL-C levels may partly explain arterial wall changes independently of insulin values. Similarly, different lipid ratios such as total cholesterol/HDL-C and LDL-C/HDL-C ratios were significantly associated with a higher prevalence of CAP.23 In agreement with those studies, although specifically analyzing middle-aged postmenopausal women in primary prevention, our data also showed a significant association between the different lipid markers and the presence of CAP.
The occurrence of IR after menopause could also contribute to the occurrence or progression of subclinical atheromatosis. For example, the estimation of HOMA-IR predicted the presence of plaques in the carotid and femoral arteries.24
The TG/HDL-C ratio is also regarded as a surrogate for IR. Masley et al. found this ratio to be associated with greater carotid intima-media thickening.16 However, this study adjusted the association for sex and age only, did not assess CAP, and analyzed younger women than those in our study. We found a significant association, irrespective of classical risk factors and using a more consistent marker of carotid subclinical atheromatosis, namely the presence of plaque.
In our study, ROC analysis showed that the TG/HDL-C ratio acceptably discriminates between women with or without CAP. Similar analyses, although not assessing the presence of CAP in postmenopausal women, have shown the value of the TG/HDL-C ratio for predicting gestational diabetes,25 cardiovascular risk in patients with familial hypercholesterolemia,26 the presence of atherogenic particles,27 the delayed progression of coronary atheromatosis in diabetic patients treated with pioglitazone,28 as well as which diabetic patients might reduce their glucose-lowering medication upon weight reduction.29 In this regard, the originality of our study lies in the fact that this particular group of patients was assessed in terms of the search for an association between the TG/HDL-C ratio and the presence of CAP.
Our analysis showed that the optimal cut-off point of the TG/HDL-C ratio for discriminating women with or without CAP was close to 2. However, as the majority of our population was considered to be at low risk according to the Framingham score, such a cut-off value may represent an underestimation.
In support of this cut-off point, Di Bonito et al. showed in a pediatric population that subjects with a TG/HDL ratio≥2.0 had a two or three-fold greater risk of hepatic and cardiac subclinical changes.30 A Swedish epidemiological study also showed that, taking as reference diabetic patients with normal weight and a TG/HDL ratio<1.9, obese diabetic subjects with a TG/HDL ratio < or ≥1.9 had a 20% and 70% greater risk of coronary disease respectively.31
The transition from premenopausal to postmenopausal status is likely to include multiple pathophysiological changes that increase cardiovascular risk factors and are partly responsible for the occurrence of subclinical cardiovascular disease. The association found in our study between IR as quantified by the TG/HDL-C ratio and the presence of CAP is consistent with such a hypothesis.
Since ultrasonographic detection of CAP is an independent predictor of cardiovascular risk, the direct relationship between different TG/HDL-C values and the prevalence of CAP seen in our study strongly suggests that the TG/HDL-C ratio is a surrogate marker of cardiovascular disease in postmenopausal women.
Our study has some limitations. First, as in any cross-sectional study, the possibility of bias (mainly selection bias) potentially influencing the results cannot be ruled out. In addition, waist circumference was not measured in our study, and the prevalence of metabolic syndrome was not estimated, so that no association could be established between the TG/HDL-C ratio and those variables. Finally, the TG/HDL ratio was selected for our study as a marker of IR because it can be easily calculated using laboratory values usually requested by healthcare professionals to stratify cardiovascular risk using risk scores. Insulin levels and HOMA were not determined because of the variability in their estimation. In our study we were not concerned with comparing the different IR markers.
In conclusion, in middle-aged postmenopausal women in primary prevention, IR, estimated from the TG/HDL-C ratio, was independently associated with a greater chance of CAP. A value of the ratio greater than 2 can be used to assess cardiovascular risk in this particular group of women.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Masson W, Siniawski D, Lobo M, Molinero G, Huerín M. Asociación entre la razón triglicéridos/colesterol HDL y ateromatosis carotídea en mujeres posmenopáusicas de mediana edad. Endocrinol Nutr. 2016;63:327–332.