To assess the efficacy of intermittent, high-dose treatment with intravenous glucocorticoids (IV GCs) in moderate to severe Graves’ ophthalmopathy (GO).
Materials and methodsPatients with GO treated with IV GCs from August 2007 to August 2011 at the Endocrinology Department of Reina Sofía Hospital were enrolled into the study. IV pulse prednisolone (7.5mg/kg/day) was administered twice weekly every two weeks for 6 weeks, and at half the dose for 6 additional weeks.
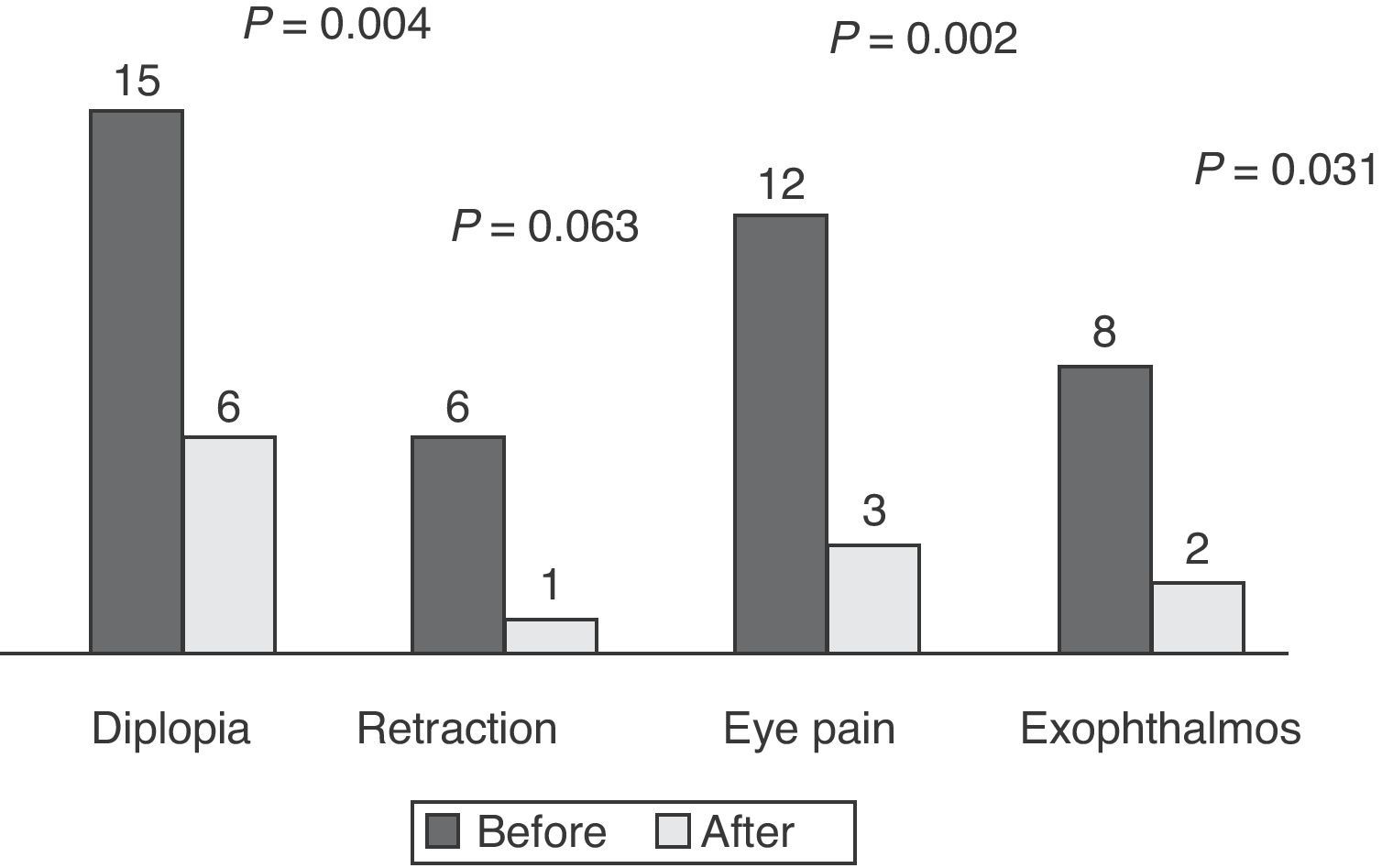
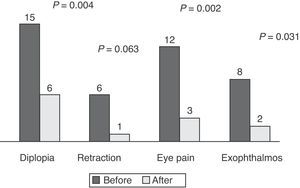
ResultsEighteen patients (mean age, 43±11 years) with moderate to severe GO were analyzed (83.3% females). Four were active smokers, five former smokers, and the rest had never smoked. Hyperthyroidism due to Graves’ disease was found in 66.7% of patients, 41.6% of whom had received radioiodine therapy. Response to treatment was satisfactory in 72.2%, partial in 11.1%, and poor in 16.7%. Mild side effects were reported by 5 patients. Before treatment, 83.3% had diplopia, 33.3% eyelid retraction, 72.2% eye pain, and 44.4% exophthalmos. After treatment, only 33.3% had diplopia (P=.004), 5.6% eyelid retraction (P=.063), 16.7% eye pain (P=.002), and 11.1% exophthalmos (P=.031). Response to treatment was not related to the underlying disease (P=.866), prior radioiodine treatment (P=.447), or smoking status (P=.368).
ConclusionsIntravenous glucocorticoid therapy decreased activity in patients with moderate to severe active GO, with major improvement occurring in diplopia, eye pain, and exophthalmos. Side effects were mild and uncommon. Treatment response was independent from the underlying disease, prior radioiodine treatment, or smoking status.
Evaluar la efectividad del protocolo de tratamiento intermitente con glucocorticoides a altas dosis por vía intravenosa (i.v.) en la oftalmopatía de Graves (OG) moderada-grave del Hospital Reina Sofía.
Material y métodosSe incluyeron los pacientes con OG tratados con glucocorticoides i.v. en nuestro servicio desde agosto de 2007 a agosto de 2011. Se administró prednisolona i.v. en dosis de 7,5mg/kg/d 2d alternos durante 6 semanas alternas y mitad de la dosis durante 6 semanas más.
ResultadosAnalizamos 18 pacientes (83,3% mujeres) con una edad media de 43±11 años. Cuatro eran fumadores, 5 habían dejado el hábito y el resto nunca habían fumado. El 66,7% presentaban hipertiroidismo por enfermedad de Graves, de los cuales el 41,6% habían recibido radioyodo. La respuesta al tratamiento fue buena en el 72,2%, parcial en 11,1% y mala en 16,7%. En 5 aparecieron efectos secundarios leves. Antes del tratamiento el 83,3% presentaron diplopía, el 33,3% retracción palpebral, el 72,2% dolor ocular y el 44,4% exoftalmos. Después del tratamiento solo el 33,3% continuaron con diplopía (p=0,004), el 5,6% con retracción palpebral (p=0,063), el 16,7% con dolor ocular (p=0,002) y el 11,1% con exoftalmos (p=0,031). El 22,2% precisaron radioterapia. La respuesta al tratamiento no se asoció a la enfermedad de base (p=0,866) al haber recibido radioyodo previo como tratamiento del hipertiroidismo (p=0,447) o al ser fumador (p=0,368).
ConclusionesEl tratamiento con glucocorticoides i.v. en la oftalmopatía tiroidea reduce significativamente la diplopía, el dolor ocular y el exoftalmos. Los efectos secundarios son leves y poco frecuentes. La respuesta al tratamiento es independiente de la enfermedad de base, de haber recibido radioyodo y de ser fumador.
Graves’ ophthalmopathy (GO) is the most common extrathyroid manifestation of autoimmune thyroid disease. The condition does not always occur in hyperthyroid patients. Up to 10% of patients affected are euthyroid and/or hypothyroid.1
GO is a rare disease that occurs in 50% of patients with Graves’ disease, but is only clinically relevant in 20–30% of those affected, of whom only 5% experience vision loss.
The etiopathogenesis of GO is not well known, and multiple studies have shown high levels of anti-TSH receptor antibodies (TSI) in the orbit of patients with GO. Autoimmunity is probably mediated by autoreactive T cells that react to one or several antigens presented in the thyroid gland and the orbit (receptors of TSH or insulin-like growth factor-I [IGF-I]), triggering a cascade which includes the secretion of different cytokines that stimulate the proliferation of orbital fibroblasts, adipose tissue expansion, and the secretion of hydrophilic glycosaminoglycans by fibroblasts. B cells are also implicated. They act as antigen-presenting and autoantibody-producing cells. The genetic factors are not known. All these molecular changes increase orbital content, which accounts for many GO symptoms, including exophthalmos, diplopia due to the dysfunction of extraocular muscles, esthetic changes, and optic nerve compression.
GO progression is influenced by potentially controllable environmental factors such as smoking, thyroid function, and the different treatment modalities.1
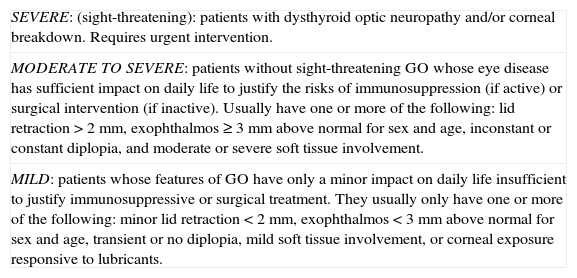
The specific treatment of GO depends on both the degree of activity (Table 1) and severity (Table 2) of the disease.2,3 Severe GO compromising vision requires urgent intervention, either surgery or IV glucocorticoids. For moderate to severe GO, glucocorticoids are indicated in the event of active disease, or surgery for inactive disease. Mild GO only requires local measures.
Clinical activity score for Graves’ ophthalmopathy. The presence of three or more symptoms suggests active disease.
| Spontaneous retrobulbar pain |
| Pain on eye movements |
| Eyelid erythema |
| Conjunctival injection |
| Chemosis |
| Swelling of the caruncle |
| Eyelid edema or fullness |
Source: Bartalena L and Tanda ML.1
Assessment of the severity of Graves’ ophthalmopathy according to EUGOGO criteria.
| SEVERE: (sight-threatening): patients with dysthyroid optic neuropathy and/or corneal breakdown. Requires urgent intervention. |
| MODERATE TO SEVERE: patients without sight-threatening GO whose eye disease has sufficient impact on daily life to justify the risks of immunosuppression (if active) or surgical intervention (if inactive). Usually have one or more of the following: lid retraction>2mm, exophthalmos≥3mm above normal for sex and age, inconstant or constant diplopia, and moderate or severe soft tissue involvement. |
| MILD: patients whose features of GO have only a minor impact on daily life insufficient to justify immunosuppressive or surgical treatment. They usually only have one or more of the following: minor lid retraction<2mm, exophthalmos<3mm above normal for sex and age, transient or no diplopia, mild soft tissue involvement, or corneal exposure responsive to lubricants. |
Source: Bartalena L et al.3
The aim of glucocorticoid treatment in GO is to decrease orbital inflammation and congestion. Systemic glucocorticoids improve GO symptoms and quality of life. They are effective provided treatment is started in the early stages of active disease. Response to glucocorticoid treatment is inversely proportional to disease duration. In GO, IV glucocorticoids have been shown to be safe and effective, with a lower recurrence rate and less side effects than oral glucocorticoids.1,2
Our objective was to assess the effectiveness of intermittent treatment of moderate to severe GO with high-dose IV corticoids at Hospital Universitario Reina Sofía (based on the protocol of the European Group on Graves’ Orbitopathy [EUGOGO]).3
Material and methodsAll patients with moderate to severe active GO according to the 2008 EUGOGO criteria treated with IV glucocorticoids at the department of endocrinology and nutrition of Hospital Universitario Reina Sofía (Cordova) from August 2007 to August 2011 were enrolled into a retrospective, observational study.3 These criteria require patients to have no severe ophthalmopathy, but an ocular disease affecting daily life to such an extent that the risk of immunosuppression if the disease is active is justified (clinical activity score [CAS]≥3 out of 7).
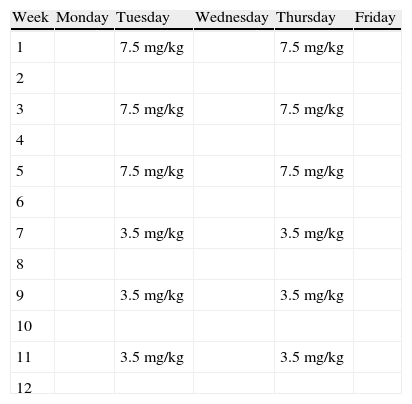
The treatment protocol consists of two phases. In the first phase, prednisolone 7.5mg/kg is administered diluted in 250mL of physiological saline over 90min twice weekly for six alternate weeks. In the second phase, half the dose (3.5mg/kg) is administered from the seventh week on two alternate days for another six alternate weeks. Table 3 shows an example.4,5 Biochemical control tests including glucose, electrolytes, transaminases, kidney function, and thyroid hormones are performed every two weeks. Blood pressure is measured before and after drug administration. In patients with diabetes mellitus, capillary blood glucose control is intensified and the insulin scheme is adjusted if required.
Treatment protocol.
| Week | Monday | Tuesday | Wednesday | Thursday | Friday |
| 1 | 7.5mg/kg | 7.5mg/kg | |||
| 2 | |||||
| 3 | 7.5mg/kg | 7.5mg/kg | |||
| 4 | |||||
| 5 | 7.5mg/kg | 7.5mg/kg | |||
| 6 | |||||
| 7 | 3.5mg/kg | 3.5mg/kg | |||
| 8 | |||||
| 9 | 3.5mg/kg | 3.5mg/kg | |||
| 10 | |||||
| 11 | 3.5mg/kg | 3.5mg/kg | |||
| 12 |
Consists of two phases: in the first phase, prednisolone 7.5mg/kg is administered twice weekly for six alternate weeks. In the second phase, half the dose (3.5mg/kg) is administered from the seventh week on two alternate days for another six alternate weeks.
All patients signed an informed consent before the start of treatment.
Demographic variables (sex and age), the cause of GO, and risk factors for GO (smoking, radioiodine treatment, and thyroid function) were collected. A comparison was made of symptoms before and after treatment with IV glucocorticoids (eye pain, exophthalmos, eyelid retraction, and diplopia) and overall subjective treatment response assessed by both patient and endocrinologist, because many patients had not been assessed by the ophthalmology department at the time of revision. Any association with radiotherapy and the presence or absence of side effects was also recorded. Any association between smoking, prior radioiodine treatment for hyperthyroidism, and the cause of the disease, i.e. Graves’ disease or Hashimoto thyroiditis, and the response to treatment with IV glucocorticoids was assessed.
In statistical analysis performed with SPSS, Chi-square statistics for contingency tables and a McNemar test for comparison before and after clinical signs were obtained. A value of P<0.05 was considered statistically significant.
ResultsEighteen patients were analyzed, of whom 83.8% were women, with a mean age of 43.17±11.12 years. Twelve patients (66.7%) had hyperthyroidism due to Graves’ disease, and the remaining six patients had hypothyroidism due to Hashimoto thyroiditis. Of patients with hyperthyroidism, five had received radioiodine therapy (41.6%), four were active smokers, five had given up smoking in the four months prior to treatment start, and the remaining patients had never smoked.
At the beginning of treatment, 15 patients had constant or inconstant diplopia, six eyelid retraction, 12 eye pain (spontaneous retrobulbar or with eye movements), and eight exophthalmos (≥3mm above normal for sex and age). Fig. 1 shows the presence of symptoms before and after treatment. Symptom disappearance was significant in all patients, except for eyelid retraction (P=0.063).
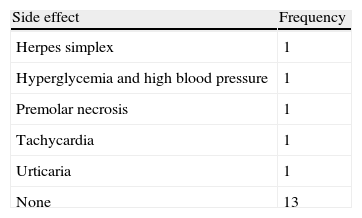
Treatment response was good (the disappearance of at least three of the four symptoms assessed) in 72.2% of patients, partial (disappearance of at least two of the five symptoms assessed) in 11%, and poor in 16.7%. Five patients (27.8%) experienced mild side effects (Table 4), and treatment was discontinued in a single patient with no symptom improvement due to tachycardia and high blood pressure two weeks after treatment start.
Retro-orbital radiotherapy was required in 22.2% of patients after treatment or during corticoid administration due to partial improvement or worsening upon corticoid tapering or discontinuation.
Response to treatment was not associated with smoking, (P=0.368), the underlying disease (Graves versus Hashimoto; P=0.866), or prior radioiodine treatment (P=0.447), nor with prior radiotherapy (P=0.226).
DiscussionIn our series, treatment of thyroid ophthalmopathy with IV glucocorticoids was effective, significantly decreasing diplopia, eye pain, and exophthalmos. While eyelid retraction improved in many patients, improvement was not statistically significant.
The European Group on GO (EUGOGO) is a multidisciplinary consortium of clinicians from different centers who share a commitment to improve the treatment of patients with GO, for which purpose they have prepared a consensus document on its clinical management and treatment.3 This document advises the use of the “clinical activity score” and “GO severity assessment” for clinical evaluation and subsequent monitoring of the patient. The EUGOGO also recommends the use of IV glucocorticoid pulses as the treatment of choice for moderate to severe GO, because this modality has been shown to decrease both the number and the severity of the side effects of glucocorticoids. The EUGOGO consensus recommends a cumulative dose of less than 8g per course of treatment and the discontinuation of IV glucocorticoids if no response occurs after the first or second week of treatment. The exact dose of prednisolone that has the therapeutic effects sought without causing adverse effects is not known. In order to assess this dose, the EUGOGO group undertook a multicenter, randomized study for which patient recruitment has recently been completed. This study compares the effectiveness of three regimens with different doses (cumulative doses of 2.5g, 5g, and 7.5g) whose data are pending publication.
A study has been published comparing two regimens. The first consisted of six doses of 0.5g/week, followed by six doses of 0.25g/week with a cumulative dose of methylprednisolone 4.5g IV, and the second included four cycles of 15mg/kg, followed by four cycles of 7.5mg/kg for a cumulative dose of 9g. A lower recurrence rate was seen with the first regimen.3,6,7
The cumulative dose was similar in our study (6.75g), but was administered twice weekly on alternate weeks. The response was good in 72% of patients and partial in 11.2%. These results were very similar to those reported in the literature, which ranged from 60% to 77%.8,9
Treatment was safe, with a complication rate of 16.7%, lower than that reported (18–56%).5 In our series, transaminase levels elevated to less than twice the upper limit of our normal ranges occurred in two patients, but resolved spontaneously. Other side effects included hyperglycemia and high blood pressure in one patient and tachycardia in another patient. These may be explained by the effect of glucocorticoids, and were resolved by adjusting antihypertensive treatment and insulin. One case each of herpes simplex and premolar necrosis, which could have been due to the immunosuppression potentially caused by glucocorticoids, was recorded. An additional patient experienced mild urticaria consistent with pruritus and erythematosus skin lesions in the trunk a few hours after the first dose of IV corticoids. This was self-limited and disappeared without treatment, but occurred again in a more severe form when treatment was restarted two weeks later.
The baseline characteristics of our population were similar to those reported in the literature. Some studies have reported a better response to glucocorticoid treatment in women and patients under 50 years of age which could be related to less severe ophthalmopathy. Two patients in our series who did not respond to treatment were males, with the difference being statistically significant (P<0.05).
The proportion of patients with GO and Hashimoto thyroiditis was 33.3%, three times greater than that reported in previous series (less than 10%),8 despite which treatment response was similar, which suggests that GO etiology is not relevant in treatment response, probably because the pathogenetic mechanism is the same.
Treatment response in our series was independent of smoking and prior radioiodine treatment. The small sample size does not allow us to draw conclusions which, moreover, do not appear to agree with reports in other publications. The patients who received retro-orbital radiotherapy did so after the glucocorticoid pulses. These were the patients with a partial response to glucocorticoids, and they did not experience a greater improvement than those not administered radiotherapy.
In addition to sample size, another limitation of our study was that the “clinical activity score” was not accurately quantified according to EUGOGO criteria at the beginning of treatment.10 Response was assessed based on patient clinical improvement and examination, although this was not expressed in grades. The clinical activity score was not used for several reasons. One reason was that most patients had not been assessed by the ophthalmologist for care reasons. Another reason was that this was a retrospective study and not all symptoms were recorded in the history, which is known to be a potential limitation. However, symptoms such as continuous or transient diplopia or exophthalmos, which are part of the EUGOGO severity scale, were recorded. Eye pain, either spontaneous retrobulbar or with eye movements, is part of the CAS scale. Proptosis was not used because it cannot be measured with the exophthalmometer. Change in eyelid retraction was not quantified because it would have involved measurement in millimeters of the distance between the lower eyelid margin and the pupil. Instead of this, we used subjective patient assessment, mainly based on nocturnal eyelid closure.
We intend to design a prospective study based on these conclusions taking into account the EUGOGO criteria.
In conclusion, our study supports the effectiveness of the administration of IV corticoids in alternate regimens as first line treatment for active, severe GO. In our hands, this protocol was safe and well tolerated by patients.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Alhambra Expósito MR, et al. Evaluación de la efectividad del tratamiento con glucocorticoides intravenosos en la oftalmopatía de Graves. Endocrinol Nutr. 2013;60:10–4.