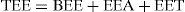Obesity has become a serious health problem worldwide because it is closely related to the leading causes of morbidity and mortality, including diabetes mellitus, hypertension, atherosclerosis, and dyslipidemia. It is therefore imperative for the scientific community to understand the main environmental and social-cultural factors, as well as organic disorders arising from breakdown of the physiological mechanisms that control energy balance in our body, all of which are ultimately responsible for the development of obesity. Adequate understanding of the mechanisms involved in regulation of energy balance is therefore essential to understand the pathogenesis and pathophysiology of the growing pandemic of obesity. This study was intended to review the main factors involved in the development of obesity and advances in understanding of the mechanisms that regulate body weight and appetite and their pathophysiology.
La obesidad se ha convertido en un serio problema de salud a nivel mundial, por su estrecha vinculación con las principales causas de morbimortalidad, diabetes mellitus, hipertensión arterial, aterosclerosis y dislipemias. En este sentido, resulta de imperiosa necesidad para la comunidad científica conocer los principales factores ambientales y socioculturales, así como los desórdenes orgánicos originados a partir de la ruptura de los mecanismos fisiológicos que controlan el equilibrio energético en nuestro organismo, y que son en última instancia responsables todos ellos del desarrollo de obesidad. Luego, una adecuada comprensión de los mecanismos involucrados en la regulación del balance energético constituye la clave para comprender la etiopatogenia y fisiopatología de la creciente pandemia de obesidad. El objetivo de este trabajo ha sido revisar los principales factores implicados en el desarrollo de la obesidad, así como los avances en la comprensión de los mecanismos reguladores del peso corporal, el apetito y su fisiopatología.
Obesity is a common pathological process in humans. Obesity has continued to exist for centuries due to genetic and environmental factors, and has now become a pandemic with serious consequences for health.1
If the human figure is considered from a plastic viewpoint through the ages, we can see that the oldest works of art include representations of the female human figure in a state of maximum adiposity. The so-called paleolithic Venus figurines, such as the Venus of Willendorf, are representative female figures for which Leroi-Gourhan (1965)2 developed a theory about the anatomical structure with which they were represented. This author stated that the body dimensions of all of them responded to a diaphragmatic organization where the vertical axis predominated as a diamond and the breasts, abdomen, and buttocks were hypertrophic, forming a circular structure in the middle of the diamond. Today, if this type of body habitus represented in art since prehistory is extrapolated to the study of obesity in humans, it is seen to be close to the current obesogenic models and archetypes.3
According to data from the World Health Organization, there are more than one thousand million people with overweight worldwide. Of these, 300 million may be considered to be obese.4 In Europe, one out of every six boys, girls, and adolescents are overweight (20%), while one out of every 20 is obese (5%).4
This increased prevalence of obesity is related to dietary factors and increasingly sedentary lifestyles.5 Increased consumption of saturated fat and carbohydrates, combined with decreased vegetable intake and low physical activity levels, are the most significant reasons for this global health problem.6 On the other hand, the consequences of obesity reach catastrophic proportions. Among such consequences, mention should be made of cardiac and vascular diseases; lipid metabolism changes (dyslipidemia), with resultant atherosclerosis; changes in certain hormones with great metabolic activity such as insulin, resulting in problems of cell resistance to insulin (hyperinsulinemia); decreased glucose tolerance, which causes a risk of type 2 diabetes mellitus; high blood pressure with the attendant risk of stroke; growth hormone deficiency, and hyperleptinemia. These factors or changes are collectively known as metabolic syndrome, and some of them may already occur at six years of age in obese children and adolescents.7
Research conducted in recent years has revolutionized our understanding of the physiological and molecular mechanisms regulating body weight.8 The discovery of leptin, its receptor, the melanocortin receptor, as well as the discovery of the action of some hormone mediators involved in the maintenance of body weight, has contributed to a better understanding of the physiological processes involved in the development of obesity.8
The etiopathogenesis of obesityThe current understanding of genetics and molecular biology allows us to consider the etiopathogenesis of obesity as a complex phenomenon.9 In this regard, the theory of a sustained increase in intake associated with deficient energy expenditure is excessively simplistic, because obesity has a very heterogeneous origin, and a variety of both genetic and nutritional factors are involved in its development.10,11
Genetic factorsRecent studies suggest that the development of obesity may have its origin in the earliest stages of life, i.e. during the fetal period. According to this, a programming mechanism would activate during the fetal period many nutritional, hormonal, physical, and psychological processes which would act in critical periods of life to shape certain physiological functions.12 The presence in the same family of one or several members with severe obesity has suggested the probable implication of genetic factors in the occurrence of this condition at an early age, an implication which has already been verified by different studies. Thus, a sevenfold greater lifetime risk of suffering extreme obesity (BMI>45) has been demonstrated when one of the parents is obese.13–15 Moreover, family studies have shown heritability indices of total body fat ranging from 20% to 80%.16 As regards the pattern of body fat distribution, estimated heritability ranges from 28% to 61% for the waist–hip ratio, and from 29% to 82% for abdominal circumference.17
According to the seventh review of the human obesity map, using data collected up to 2005, 47 cases of monogenic obesity, 24 cases of Mendelian changes, and 115 different loci susceptible of being involved in polygenic obesity have been reported. In this regard, the obesity map suggests that, except in the Y chromosome, all chromosomes have genes with a potential implication in obesity occurrence and development.18 Today, based on the results of the 222 studies conducted on genes and obesity, there is adequate scientific evidence available to identify 71 genes as potential inducers of the occurrence of obesity.19 If chromosomal regions are also considered, this number increases to more than 200 genes. Fifteen of these genes are closely associated with body fat volume.20 One of the genes discovered with a potential implication in the development of obesity at an early age is the FTO gene.21 This gene is considered to induce progressive weight gain in subjects in whom it is overexpressed.22 Its expression is usually greater in the hypothalamic areas involved in the feeding process.23 It has also been noted that in the event of acute food deprivation, expression of this gene is modified, which suggests its potential interrelationship at the level of appetite and satiety sensations.24 Thus, data from a study conducted in children showed that a close relationship exists between the sensation of satiety reported by children and the degree of gene expression. Thus, children carrying two risk alleles showed a significantly lower satiety response.25
On the other hand, mutations in some human genes responsible for the occurrence of the pleiotropic effects associated with morbid obesity conditions as a clinical manifestation have been known since the 80s.26 One such condition is Prader–Willi syndrome, an autosomal dominant disorder. Seventy percent of patients with this syndrome have abnormalities in several genes located in turn in chromosome 15 of the father.27 Clinically, this syndrome is characterized in children by the occurrence of obesity, muscle hypotony, mental retardation, hypogonadism, cryptorchidism, and low height associated with small hands and feet. In some cases, the syndrome is usually associated with the presence of non insulin-dependent diabetes mellitus, as well as ketogenesis and hyperglycemia.28 This syndrome represents one of the most prevalent examples of dysmorphic obesity in humans.28
Alström–Hallgren syndrome is characterized by the occurrence of blindness due to retinal dystrophy, nerve deafness, cardiomyopathy, diabetes mellitus, and renal failure, but with no polydactyly or mental retardation. In this syndrome, obesity usually occurs from two years of age, with weight often increasing to values 100% higher than the normal values for age and sex.29 Another characteristic feature of this condition is the occurrence of skin changes, mainly acanthosis nigricans, arising from the chronic association between diabetes mellitus and marked insulin resistance. Inheritance is autosomal recessive, and the syndrome is caused by a mutation in the ALMS1 gene, located in chromosome 2.30
The intestinal microbiota and its impact on obesity developmentThere is currently some controversy as to whether the microbiota colonizing the human bowel is involved or not in obesity development. The intestinal microbiota is known to play essential biochemical roles such as obtaining energy through diet and the synthesis of vitamins and other absorbable compounds.31 However, imbalances in the composition of the intestinal microbiota have been associated with the occurrence of insulin resistance and body weight increase.32 Therefore, many studies aimed at modulating the composition of the intestinal microbiota in order to control those disorders, such as obesity, with a metabolic basis have been undertaken. Thus, the Sato et al. study (2008)33 showed that the administration of milk fermented with Lactobacillus gasseri decreased the size of adipocytes in mesenteric adipose tissue and also reduced serum leptin levels. This showed the potential regulatory effect of such bacteria on adipose tissue growth and, thus, on obesity. In another study conducted by Ma et al. (2008)34 on mice fed a fat-rich diet, the administration of Lactobacillus, Bifidobacterium, and Streptococcus with diet improved steatosis and insulin resistance induced in these mice by excess fat. Studies of this type are improving our understanding of the potential relationship between metabolism and certain components of the microbiota. However, further studies are needed to answer all pending questions and ascertain the factors implicated in the development of obesity and its associated metabolic diseases.
Changes in dietary and physical activity patternsIn the Western world, the advent of food biotechnology has allowed for the consumption of any type of food throughout the year. This, combined with an almost unlimited access to food of a great part of the population, has resulted in a number of changes in the usual diet of individuals.
Thus, an increase has occurred in the consumption of food of animal origin and carbonated beverages, which provide 20–30% of total daily energy intake. Some studies show that excess intake of fruit juice (more than 350mL/day) may promote obesity development in pre-school children, and even limit their growth.35 It should be noted that overfeeding of children and adolescents by these products is a central element that explains excess body fat accumulation.36
González Jiménez (2010)37 suggested that total calories; food composition, palatability, and variety; and the size and number of daily meals are also factors closely related to obesity.
Other factors to be considered include current lifestyles, in which the hours of work of the parents often make the daily preparation of food difficult and cause them to resort in many cases to precooked foods,38 and carbohydrate-rich beverages such as artificial fruit juices and carbonated drinks instead of water.39 As a result, there is virtually no diet control.
Similarly, eating out permanently also contributes to a progressive increase in adipose tissue because meals taken out of home are usually rich in fat and have a high calorie content. Moreover, it should be taken into account that some children have their main meal of the day (lunch) at school, and many school canteens do not provide a healthy diet.40
Apart from the above, the generalized increase in the prevalence of obesity in the past 25 years has partly been due to a progressive reduction in physical activity levels.41 In the specific case of adolescents, these have been found to use public transport whenever they need to walk for more than 15min. According to technical data, this results in a 37% reduction in the number of trips on foot and a 20% reduction in the number of kilometers walked every year. Data from the ENKID42 study suggest that only 32.2% of boys and 17.8% of girls aged 6–9 years practice sport more than two days per week in their leisure time. In our view, this is really alarming. As regards the prevalence of sedentary lifestyles in the different Spanish regions, Andalusia and the Canary Islands are the regions where children and adolescents have the highest rates of sedentary behavior during their leisure time (64% and 68% respectively).37
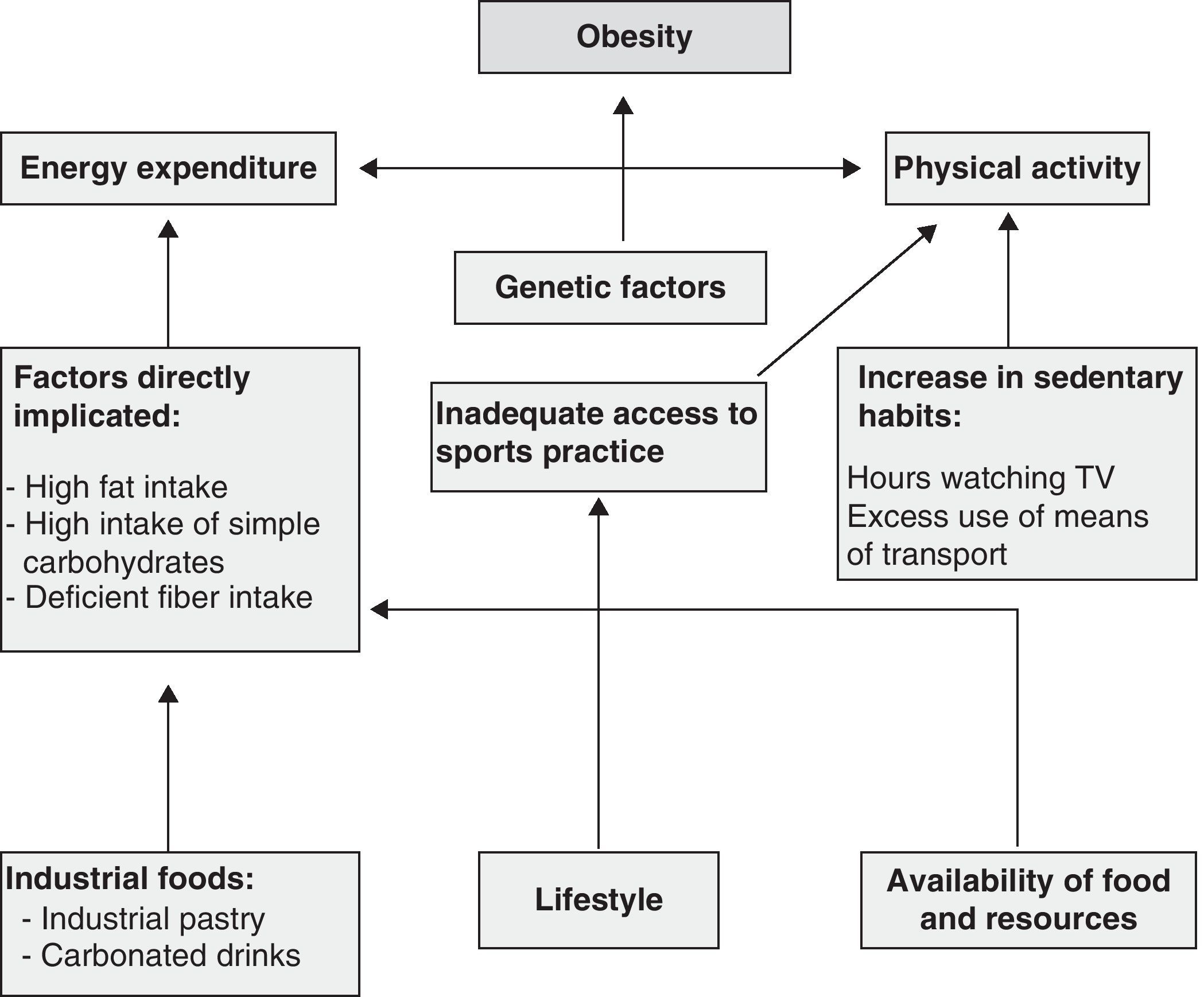
According to Stefanick,43 there is general agreement that daily physical activity is the predominant factor in the maintenance of body weight and that it is therefore important for weight loss. However, any activities considered to involve low energy expenditure deserve special consideration because in a wide majority of cases it is due to such activities that body weight regulation will be achieved and maintained.44Fig. 1 shows the factors involved in the development of obesity.
Conceptual framework of the main factors involved in obesity.
According to the first law of thermodynamics, obesity is the result of an imbalance between energy expenditure and supply. In the case of our body, this energy comes from the main essential nutrients: carbohydrates, protein, and fat.45 Carbohydrates are the first step in the supply of energy. When carbohydrate consumption exceeds the required amounts, carbohydrates are converted into fat. When carbohydrates are absent or at very low levels, fat is mobilized to be used for energy production. In this process, called lipolysis, fat is converted into fatty acids and glycerol. The human body thus meets the physical laws represented by this first principle of thermodynamics, according to which energy is neither created nor destroyed, it just changes form. Any excess energy introduced changes the internal energy of the body and is transformed into chemical energy, for which the main store is fatty tissue.46 An energy intake (EI) greater than total energy expenditure (TEE) will inevitably result in an increased adipose tissue, which is always associated with increases in lean mass and body weight, in whose control TEE plays a significant role.47
Body weight may therefore change depending on intake and total energy expenditure (TEE), which is equal to resting or basal energy expenditure (BEE) plus energy expenditure during physical activity (EEA) and energy expenditure derived from thermogenesis (EET).48 This is defined by the energy balance equation:
However, the nervous system, the gastrointestinal system including the liver and pancreas, and the adipocyte are involved in the regulation of energy expenditure and intake.49 Adipocytes are highly differentiated cells with three roles: storage, energy release, and endocrine/metabolic. Adipocytes may change 20-fold in diameter and 1000-fold in volume. Each adipocyte is able to store a maximum volume of 1.2μg of triglycerides. Two enzymes, lipoprotein lipase (LPL) and acylation stimulating protein (ASP), both activated by the action of insulin and chylomicrons, participate in this process, called esterification. The content of triglycerides stored inside the adipocyte does not usually exceed 0.6μg per cell. Since the mean number of adipocytes in each subject is 30–60×109, and each of these contains 0.5μg of triglycerides, we may infer a total fat volume of 15kg, or 135,000kcal.50
However, it was the discovery of leptin and the genes regulating its production in adipocytes that caused a revolution in our understanding of intake-expenditure regulation and, thus, in the study of obesity. Leptin is the product of the ob gene. In humans, the ob gene is located in chromosome 7q 31.3,51 has 650kb, and consists of three exons separated in turn by two introns. Exons 2 and 3 carry the region encoding for leptin synthesis.51
Biochemically, the ob gene is a peptide consisting of 167 amino acids with a similar sequence in different species, as shown by the fact that human leptin is 84% homologous to mouse and 83% homologous to rat leptin.52 Through this hormone, the hypothalamus exerts an effect to control energy homeostasis in the body, modulating intake and counteracting a potential positive energy balance.53 For this, leptin induces the activation of catabolic effector systems. These systems decrease adiposity through appetite inhibition (anorexigenic effect), thus stimulating energy expenditure and disabling the anabolic effector systems, whose purpose is to increase body adiposity (through appetite increase), thus promoting adipose tissue lipolysis.54
Leptin exerts its anorexigenic action through its receptor, located in neurons of the infundibular nucleus of the hypothalamus. Activation of the leptin receptor will trigger a complex framework of mechanisms. These include a decreased secretion of neuropeptide Y, the most potent endogenous appetite stimulant.55 Secondly, and in parallel, a decrease will occur in the secretion of agouti-related protein. This protein is an antagonist of melanocortin 1 and 4 receptors, which are in turn appetite regulators.55
Leptin, through the hypothalamus, uses the sympathetic nervous system to stimulate the release of thyroid-stimulating hormone.56 In addition, noradrenergic receptors also modulate body weight through leptin by stimulating alpha-1 and beta-3 receptors, decreasing intake and increasing energy expenditure.57
The efferent parasympathetic nervous system modulates hepatic metabolism, insulin secretion, and gastric emptying, thus participating in body weight control and regulation.
Olfactory and gustatory stimuli caused by food also participate in intake regulation. These stimuli represent peripheral signals which will in turn be integrated and processed in the nervous system, releasing neurotransmitters which will modulate intake. The most widely studied of these neurotransmitters is serotonin. Serotonin receptors regulate the amount of food ingested and the selection of macronutrients. Receptor stimulation at hypothalamic level decreases overall intake, and specifically fat intake.58
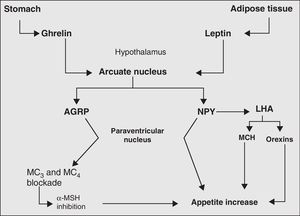
The α-melanocyte-stimulating hormone (α-MSH) is an anorexigenic peptide closely involved in intake regulation. It is synthesized in the arcuate nucleus, from which it is widely distributed by the central nervous system, especially at the paraventricular nucleus. The administration of α-MSH in the ventricular system of the brain causes appetite decrease and increased thermogenesis.59 The hormone acts in the brain by interacting with type 3 and 4 receptors (MC3 and MC4). Its effects in humans are increasingly known, and it may be one of the key elements for the treatment of obesity.59
Another biomolecule implicated in the modulation of food intake is neuropeptide Y, whose main function is to increase food intake. Neuropeptide Y is the most potent neurotransmitter with an anabolic action.60
Intestinal peptides also have a modulatory action of food intake. Thus, peptides such as cholecystokinin, gastrin-releasing peptide, and bombesin decrease food intake. Insulin has, in turn, an anabolic effect, promoting glucose uptake and lipid accumulation in tissue.61
Ghrelin, discovered by Kojima (1999),62 was the first peptide reported to have an orexigenic effect. Ghrelin acts in the hypothalamus through three pathways: first, by accessing the arcuate nucleus through the bloodstream by crossing the blood–brain barrier. A second pathway consists of vagal afferences traveling in the vagus nerve from the stomach to the hypothalamus. Finally, ghrelin is also synthesized in the hypothalamus.63 Other studies have demonstrated that plasma ghrelin levels increase during periods of negative energy balance, i.e. in fasting conditions, and subsequently normalize when food is ingested.64
Other gastrointestinal peptides involved in the satiety process include the glucose-dependent insulinotropic polypeptide (GIP), which is able to induce insulin secretion if elevated blood glucose levels occur.65 GIP receptor-deficient mice developed a phenotype resistant to diet-induced obesity, which suggests that GIP may be involved in the pathogenesis of central obesity.66 Secretion of glucagon-like peptide-1 (GLP-1) by intestinal L cells, mainly in the ileum and colon, occurs after nutrient intake (carbohydrates and fatty acids) and in proportion to calorie content. The Näslund et al. study67 showed that, in obese patients, subcutaneous administration of GLP-1 before each meal for five days decreased their food intake by up to 15%, causing a weight loss of 0.5kg. Final mention should be made of peptide YY (PYY) or tyrosine–tyrosine, a member of the pancreatic polypeptide (PP) family. Not only this is synthesized by L cells of the distal gastrointestinal tract (colon and rectum), but is also present in the stomach, the pancreas, and some regions of the central nervous system.68 PYY is secreted as a function of calorie intake, with lipids being mainly responsible for its secretion.68 Under fasting conditions, plasma PYY levels are low, but increase within 15–30min of the start of intake.69 PYY secretion and release into blood allow for nutrient absorption by delaying gastric emptying and intestinal transit.69
Glucagon is another hormone synthesized by the pancreas and also involved in the regulation of food intake. The main function of glucagon is to stimulate glycogen breakdown and the start of gluconeogenesis, thus promoting catabolism. From the exocrine viewpoint, pancreas synthesizes enterostatin (pancreatic procolipase activation peptide), whose role is to decrease fat intake and to cause satiety.70
As regards efferent body weight control systems, the significance and participation of the endocrine and neurovegetative systems should be stressed. The endocrine system is represented by growth, thyroid, and gonadal hormones, glucocorticoids, and insulin.71
During development, growth and thyroid hormones work in concert to increase growth. Once in the blood, growth hormone stimulates the liver to produce another hormone, insulin-like growth factor-1 (IGF-1), which plays a key role in growth during childhood. Thyroid hormones act by increasing protein synthesis in all body tissues.72
Gonadal steroids start to act during pubertal development. Thus, testosterone increases lean body weight as compared to fat, while estrogens have the opposite effect. Testosterone levels decrease in males with age, which causes increases in visceral and total body fat and a decrease in lean body weight.72 Adrenal glucocorticoids have a significant action in neuroendocrine control of food intake and energy expenditure.72
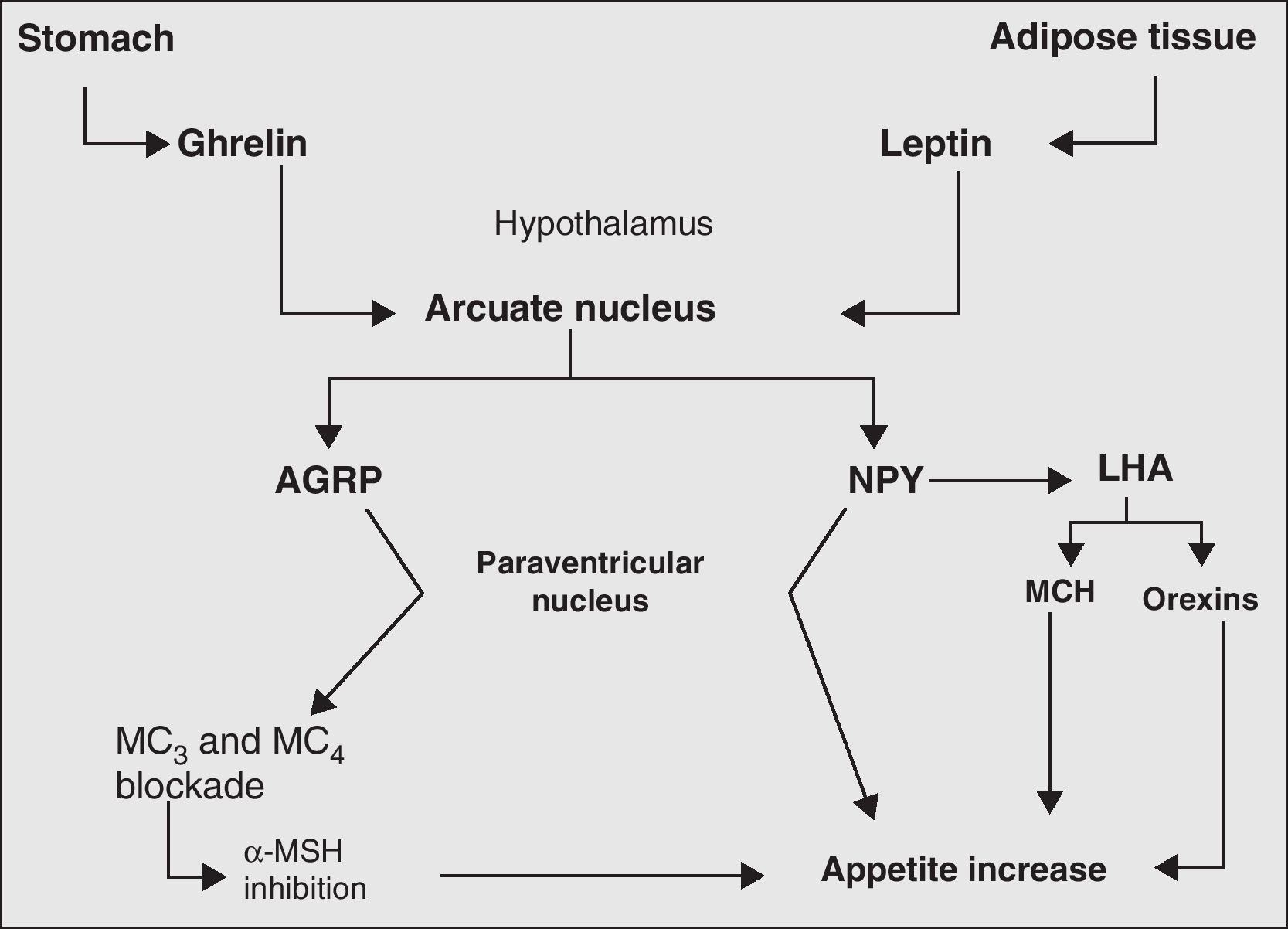
Finally, the neurovegetative system represents the final link in the chain of processes and biomolecules responsible for body weight control. Its main action is the regulation of hormone secretion and thermogenesis.73Fig. 2 more clearly depicts the mechanisms, main biomolecules, and structures of the nervous system involved in appetite regulation.
Main orexigenic mechanisms involved in appetite regulation. LHA, lateral hypothalamic area; AGRP, agouti-related peptide; MCH, melanocyte concentrating hormone; MC, MSH receptors; MSH, melanocyte-stimulating hormone; NPY, neuropeptide Y; PVN, paraventricular nucleus.
Regardless of the etiology of obesity, adequate knowledge of the mechanisms involved in the regulation of energy balance is essential for understanding the etiopathogenesis and pathophysiology of the increasing pandemic of obesity.
The two efferent systems for body weight control, the endocrine system–including growth, thyroid, and gonadal hormones, glucocorticoids, and insulin–and the neurovegetative system, jointly contribute to regulating energy balance.
Finally, the great advances in our understanding of the main biomolecules regulating body weight and food intake have significant implications for the clinical management and treatment of obesity. While diet and exercise continue to be the mainstays for obesity management, an increasing number of patients require pharmacological support to achieve or maintain body weight reduction. In this regard, intervention in certain processes or stages of the energy homeostasis system could be essential for improving health and nutritional status in these patients.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: González Jiménez E. Obesidad: Análisis etiopatogénico y fisiopatológico. Endocrinol Nutr. 2013;60:17-24.