Transsexual patients can only be diagnosed and treated at functional gender identity units with the provision of high quality care, development of clinical practice guidelines, and interdisciplinary working groups. The therapeutic process has three mainstays: initial psychological diagnostic evaluation and psychotherapy, endocrinological evaluation and hormone therapy, and sex reassignment surgery. Cross-sex hormone therapy is essential for the anatomical and psychological transition process in duly selected patients. Hormones help optimize real-life sex identity, improve quality of life, and limit psychiatric co-morbidities often associated to lack of treatment. Development of this clinical practice guideline addresses the need for implementing a coordinated action protocol for comprehensive health care for transgender people in the National Health System.
El abordaje diagnóstico-terapéutico de los pacientes transexuales solo puede desarrollarse en unidades funcionales de Identidad de Género, con la provisión de servicios de alta calidad asistencial, desarrollo de guías de práctica clínica y grupos de trabajo interdisciplinarios. El proceso terapéutico consta de 3 pilares fundamentales: evaluación diagnóstica psicológica inicial y psicoterapia, evaluación endocrinológica y terapia hormonal y cirugías de reasignación sexual. El tratamiento hormonal cruzado es un elemento importante en el proceso de transición anatómica y psicológica de los pacientes apropiadamente seleccionados. Las hormonas contribuyen a optimizar el proceso de vida real en el sexo identitario, mejoran la calidad de vida y limitan las comorbilidades psiquiátricas que muchas veces se asocian a la falta de este tratamiento. La elaboración de esta guía de práctica clínica responde a la necesidad de implantación de un protocolo de actuación coordinado para la atención sanitaria integral a las personas transexuales en el Sistema Nacional de Salud.
Transsexuality or transsexualism is considered as the most extreme situation among mismatches between gender identity and biological sex (gender identity disorder, GID), and is defined as a strong discomfort or dysphoria with one's anatomical sex and identification with the other sex. This cross-gender identity usually leads to a number of physically and socially adaptive changes in everyday life (external appearance, choice of a name consistent with identity, gender role, etc.) which constitute the test of real life or of real life experience (RLE).1
The care of patients who experience GID is an excellent example of the need for a multidisciplinary team. Patients should participate in the therapeutic and decision-making process after receiving adequate information from clinicians. The waiting time for access to this service should be in line with the delay for other services provided by a tertiary hospital, which is where the multidisciplinary unit is usually located. After initial assessment, patients should be informed of the approximate waiting times, so that they are aware that their treatment progresses according to the time schedule established in the protocol.
Diagnosis and treatment may only be performed at functional gender identity units (GIUs) in order to provide access to resources while avoiding unnecessary waiting times, as well as high quality care based on clinical practice guidelines and interdisciplinary work teams. The therapeutic process has three mainstays: initial psychological diagnostic evaluation and psychotherapy, endocrine evaluation, and hormone therapy and sex reassignment surgery (SRS). Cross-sex hormone treatment (CSHT) is an important element in the anatomical and psychological transition process of duly selected adults with GIDs. Hormones contribute to optimizing the real life process in the identity sex, improving the quality of life, and decreasing the psychiatric comorbidities often associated with a lack of hormone treatment.
These clinical practice guidelines have been prepared in order to meet the need for the implementation of a coordinated action protocol for integral health care for transsexual people in the National Health System. These guidelines are consistent with the international consensus on the management of this clinical condition.
DefinitionsThere are two international manuals used by clinical professionals which state the criteria required in order to diagnose transsexualism: the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) and the International Code of Diseases (ICD-10). They both include GIDs in their sections devoted to mental problems. This is currently a controversial issue because although most patients report adaptive difficulties and thus great suffering because of their transsexual condition, they do not have any other specific mental condition.2
GID (DSM IV). This refers to patients with strong, consistent cross-gender identification, with persistent discomfort with their sex, and a sense of inappropriateness in gender role. It is in turn subdivided according to whether it is diagnosed in children, in adolescents or in adults. People not meeting these criteria are labeled as having an unspecified GID, a category that may include GIDs commonly associated with sex differentiation disorders (formerly called intersex conditions).2
The ICD-10 includes five diagnoses within GID (F 64): transsexualism, dual-role transvestism, GID of childhood, other GIDs, and GID, unspecified.
Transsexualism (IDC-10, F64.0). Three criteria must be met: a desire to live and be accepted as a member of the opposite sex, usually accompanied by a wish to have hormonal treatment and surgery to make one's body as congruent as possible with the preferred sex; transsexual identity has been present persistently for at least two years; and the condition is not a symptom of a mental disorder or chromosome abnormality.
This is the most extreme form of sexual dysphoria, and people who suffer from it report a constant feeling and a persistent conviction of belonging to the other sex, which creates in them a permanent gender identity conflict which is the cardinal aspect of the phenomenon.
Gender dysphoria. The experience of a mismatch between sexual appearance and the personal feeling of being male of female.
Male-to-female (MTF) transsexual. MTF patients (transsexual women) feel and identify themselves as women, but are aware that they are anatomically males. Therefore, they make every possible effort to adapt their bodies to this identity. These people show a persistent concern for hiding their primary and secondary sex characteristics, and most of them request hormonal and surgical treatment to change sex.
Female-to-male (FTM) transsexual. FTM patients (transsexual men) are anatomically women but show attitudes, behavior, and interests characteristic of males. Male behavior, clothing, and posture are adopted to differing extents. These people report a strong desire to adopt the social role of men, be accepted as such, and acquire a male physical appearance. Hence, from adolescence they tend to dress in ambiguous or male clothing, wearing tight clothes to hide their breasts.
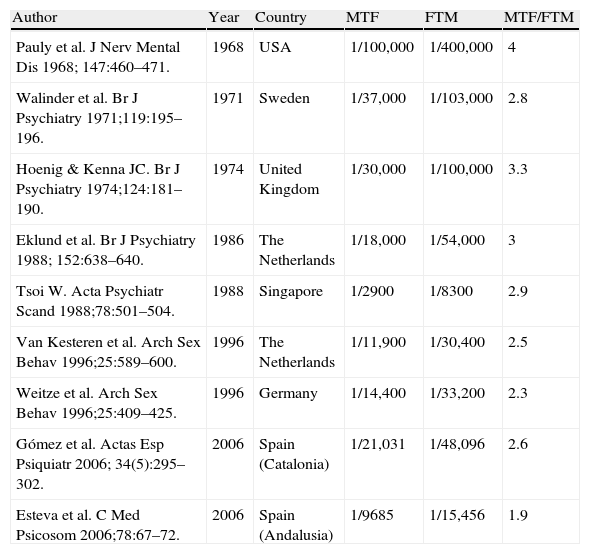
EpidemiologyWidely different estimates of GID prevalence have been reported (Table 1).3 The most recent studies in Europe reported prevalence rates of 1:12,225 inhabitants in Scotland,4 1:11,900 biological males and 1:30,400 biological females in The Netherlands,5 and 1:4000 inhabitants in England6 (Gender Identity Research and Education Society (GIRES) 2008). The MTF/FTM ratio ranges from 3:1 to 4:1. GIRES assumes a GID incidence of 3 per 100,000 of the population over 15 years of age. Prevalence rates reported in Spain range from 1:9685–1:21,031 biological males for MTF to 1:15,456–1:48,096 biological females for FTM.7–9 Extrapolation of these prevalence rates to the Spanish population gives total numbers of 3865 MTFs and 1513 FTMs, although these figures are probably underestimates (see reference on Spanish data below).
Prevalence and sex ratio of transsexualism in various national and international studies (3).
| Author | Year | Country | MTF | FTM | MTF/FTM |
| Pauly et al. J Nerv Mental Dis 1968; 147:460–471. | 1968 | USA | 1/100,000 | 1/400,000 | 4 |
| Walinder et al. Br J Psychiatry 1971;119:195–196. | 1971 | Sweden | 1/37,000 | 1/103,000 | 2.8 |
| Hoenig & Kenna JC. Br J Psychiatry 1974;124:181–190. | 1974 | United Kingdom | 1/30,000 | 1/100,000 | 3.3 |
| Eklund et al. Br J Psychiatry 1988; 152:638–640. | 1986 | The Netherlands | 1/18,000 | 1/54,000 | 3 |
| Tsoi W. Acta Psychiatr Scand 1988;78:501–504. | 1988 | Singapore | 1/2900 | 1/8300 | 2.9 |
| Van Kesteren et al. Arch Sex Behav 1996;25:589–600. | 1996 | The Netherlands | 1/11,900 | 1/30,400 | 2.5 |
| Weitze et al. Arch Sex Behav 1996;25:409–425. | 1996 | Germany | 1/14,400 | 1/33,200 | 2.3 |
| Gómez et al. Actas Esp Psiquiatr 2006; 34(5):295–302. | 2006 | Spain (Catalonia) | 1/21,031 | 1/48,096 | 2.6 |
| Esteva et al. C Med Psicosom 2006;78:67–72. | 2006 | Spain (Andalusia) | 1/9685 | 1/15,456 | 1.9 |
MTF: male-to-female transsexual; FTM: female-to-male transsexual.
(3) Esteva I et al. Transexualismo. Manual del residente en endocrinología y nutrición. 2009 (ISBN 978-84-692-1374-2).
The treatment of GID based on a combination of hormone therapy and, usually, a combination of SRS associated with adequate psychological assessment and support provides excellent results, with a rate of success, defined as personal satisfaction after the process, higher than 90% and very low rates of regret of 0.5–3%.10,11 Factors predicting for a poor prognosis and regret after irreversible changes include the loss of family and social support, personal instability, personality disorders, the presence of psychotic disorders, and the occurrence of traumatic events such as surgical complications, emotional ruptures, and work loss. Factors predicting for postoperative improvement include earlier age, accurate diagnosis of the condition, good social and psychic function before surgery, and the availability of social support.
EtiologyGender identity (the sense of being a men or a woman) gradually evolves during childhood and youth. This cognitive and affective learning process occurs in interaction with relatives, colleagues, and the environment, and it is not known when gender identity crystallizes or what factors contribute to the development of an atypical gender identity.12,13 Genetic studies of behavioral disorders in childhood suggest a hereditary component.14 However, except for gender dysphoria occurring secondarily to certain sex differentiation disorders, no clear information exists about the etiopathogenesis of sex identity change in childhood, and since in most children dysphoria does not persist during adolescence and adulthood, these data cannot be extrapolated to adults. Due to inadequate understanding of the effects of sex steroids on brain development and function, the biological bases of gender identity formation in humans have not been identified.14–16 To sum up, neither biological nor psychological studies provide a satisfactory explanation for the occurrence of this condition at these ages.
Quality of careAvailability, accessibility, resourcesThe GID unit should be competent and efficient, accessible to a given geographic area or at a reasonable travel distance that allows for adequate adherence to the process. Waiting time, access procedure, and the protocol to be used should be reported to each patient according to standards,1,17 and diagnosis and treatment should be performed at specialized units using a multidisciplinary approach (i.e. one in which all specialists involved in the process participate in physical proximity to each other and discuss the cases studied and monitored in clinical sessions). The implication of family and community medicine and mental health specialists of the healthcare area through the development of training activities is also important.
Patient and flexibilityHealthcare professionals should respect at all times the autonomy of the patient for taking decisions at each stage of the process. Treatment should be focused on the individual patient, recognizing the individual's preferences, needs, and circumstances. Treatment should not be prescribed immediately. Rather, subjects should be offered different adequate options and should be informed of the resultant benefits and risks for their health. Agreement should be reached on treatment progression and time sequence within established standards. Informed consent for each of the steps in the process is essential for this. If physician and subject disagree, the patient should be entitled to obtain a second independent opinion from another specialist working in this field.
Clinical protocolTo assess people with GIDs, it is recommended that the international standards of the Harry Benjamin International Gender Dysphoria Association (HBIGDA) (currently called the World Professional Association for Transgender Health, WPATH)1 and The Endocrine Society be followed.16 These recommendations provide the internationally agreed parameters and instructions for the diagnosis and treatment of GID. As discussed above, treatment should be performed at specialized units and using a multidisciplinary approach.2 Within these multidisciplinary teams, mental health specialists (psychologists and psychiatrists) are responsible for confirming initial diagnosis and for psychotherapeutic management during the sex reassignment process (SRP). Patients receive a three-stage treatment (the so-called therapeutic triad) consisting of: (1) diagnosis and RLE, (2) CSHT, and (3) SRS.
Diagnosis and real life experienceInitial evaluation. Psychotherapy and therapeutic supportNot all people who request sex reassignment are transsexuals.18 Transsexualism is not uncommonly confused with other forms of GID and other conditions not amenable to hormone or surgical treatment. Diagnostic evaluation is a long, complex process that should be strictly controlled, extensive and last for as long as required.2,18–21 GID may be associated with psychological or psychiatric problems.22–24 The diagnosis of GID is the responsibility of mental health professionals (MHPs). This initial stage should be followed, throughout the sex change process, by regular evaluation of the case, psychotherapy and family therapy if deemed necessary, and coordination with the rest of the team (endocrinologists and surgeons). Diagnostic evaluation requires at least 4–6 months, during which regular contact should be maintained with the professional responsible. Adequate differential diagnosis is required, because lack of certainty at this stage is associated with increased regret after sex reassignment treatment and is a negative predictor of the subsequent outcome.10,25
Real life experienceOnce a certain diagnosis is made, and if patients have not already started RLE, the convenience of starting this in the different social environments within which they interact is recommended, except in clearly difficult and hostile situations. RLE means that the person lives, works, and relates in all his/her life activities according to the desired sex and for the longest possible time.
Before any SRS procedure, a MHP must re-evaluate the situation, which will allow for confirming the diagnosis of GID and successful completion of RLE. For mastectomy in FTMs, the 12–24 months of hormone therapy need not be completed before surgery is indicated, because the presence of breasts may make RLE progression unfeasible. However, a confirmatory diagnosis and the start of CSHT at least six months before are indispensable.
Endocrine assessment and hormonal interventionInitial evaluationThis includes a complete clinical history, lifestyle habits, family history of neoplasms, early cardiovascular disease and thrombotic events, surgical procedures; pubertal development and gonadal function history; the prior use of CSHT (drug name and dose and treatment duration) and the provision of supplemental laboratory tests performed before the start of such therapy, if available; the clinical results collected and the degree of satisfaction achieved, also recorded in this part of the patient's history; the procedures used to eliminate secondary sexual characteristics, such as depilation, electrolysis, and plasties.2
During the interview, the self-referential language used by patients and their social and work situation and family and social support should be noted, as well as data about their real life tests and strategies for adapting to their situation.
The possibility that these patients have secondary transsexualism due to congenital adrenal hyperplasia, virilizing tumor, androgen resistance, chromosomal disease, testicular agenesia, or hypogonadism of any type that may have caused their GID should be explored in the interview.2 These are uncommon conditions, but require a different diagnostic and therapeutic approach.26
The following should be performed: physical examination, secondary sexual characteristics (Tanner stages), breast examination, genital examination (including testicular volume and length of penis), anthropometric measures, and blood pressure values; cardiopulmonary auscultation; abdominal examination; signs of chronic venous insufficiency; waist/hip index and bioimpedanciometry where available.
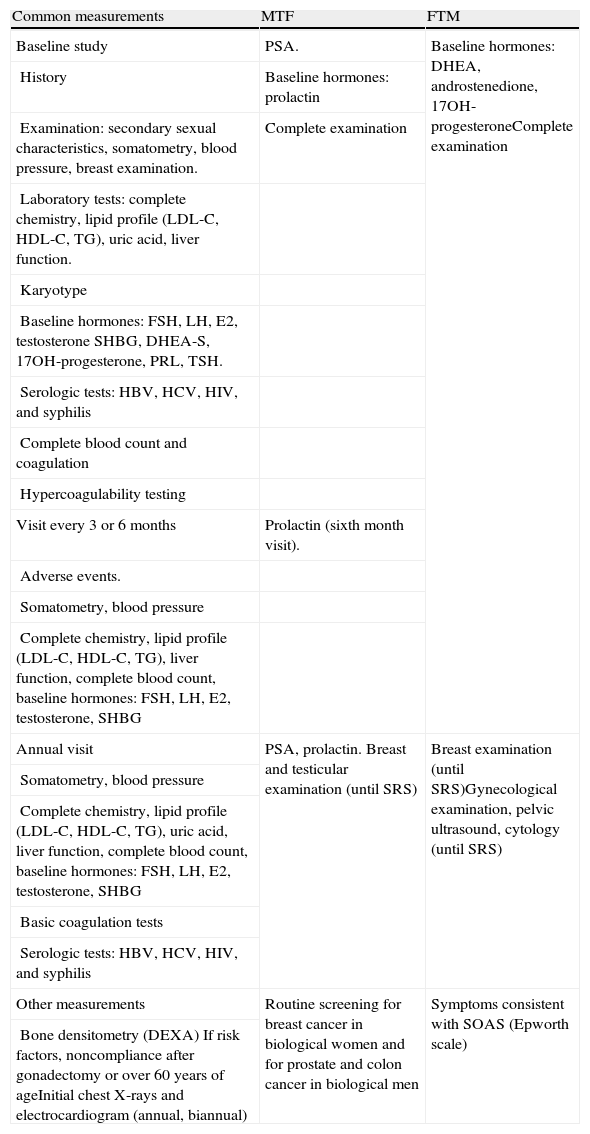
Supplemental examinations are performed in order to rule out the presence of any hormonal or chromosomal changes that may cause behavioral disorders, and also of other associated conditions that could contraindicate or condition hormone therapy. Recommended tests include general chemistry, liver function tests, complete lipid panel, uric acid, prostate-specific antigen, complete blood count, karyotype, and baseline hormone tests (Table 2). In the event of unsupervised sex hormone intake, a washout period of at least one month (depending on the preparation taken) is necessary before the supplemental tests are done.27
Initial assessment and monitoring of hormone therapy in adults with mismatch between gender identity and biological sex (29).
| Common measurements | MTF | FTM |
| Baseline study | PSA. | Baseline hormones: DHEA, androstenedione, 17OH-progesteroneComplete examination |
| History | Baseline hormones: prolactin | |
| Examination: secondary sexual characteristics, somatometry, blood pressure, breast examination. | Complete examination | |
| Laboratory tests: complete chemistry, lipid profile (LDL-C, HDL-C, TG), uric acid, liver function. | ||
| Karyotype | ||
| Baseline hormones: FSH, LH, E2, testosterone SHBG, DHEA-S, 17OH-progesterone, PRL, TSH. | ||
| Serologic tests: HBV, HCV, HIV, and syphilis | ||
| Complete blood count and coagulation | ||
| Hypercoagulability testing | ||
| Visit every 3 or 6 months | Prolactin (sixth month visit). | |
| Adverse events. | ||
| Somatometry, blood pressure | ||
| Complete chemistry, lipid profile (LDL-C, HDL-C, TG), liver function, complete blood count, baseline hormones: FSH, LH, E2, testosterone, SHBG | ||
| Annual visit | PSA, prolactin. Breast and testicular examination (until SRS) | Breast examination (until SRS)Gynecological examination, pelvic ultrasound, cytology (until SRS) |
| Somatometry, blood pressure | ||
| Complete chemistry, lipid profile (LDL-C, HDL-C, TG), uric acid, liver function, complete blood count, baseline hormones: FSH, LH, E2, testosterone, SHBG | ||
| Basic coagulation tests | ||
| Serologic tests: HBV, HCV, HIV, and syphilis | ||
| Other measurements | Routine screening for breast cancer in biological women and for prostate and colon cancer in biological men | Symptoms consistent with SOAS (Epworth scale) |
| Bone densitometry (DEXA) If risk factors, noncompliance after gonadectomy or over 60 years of ageInitial chest X-rays and electrocardiogram (annual, biannual) |
DEXA: dual energy X-ray absorptiometry; E2: estradiol; TT: total testosterone; PSA: prostate-specific antigen; SRS: sex reassignment surgery; SOAS: sleep obstructive apnea syndrome; male-to-female transsexual; FTM: female-to-male transsexual.
(29) Modified from: Moreno-Pérez O. Transexualidad: control del tratamiento, en Función androgénica en el laboratorio. Edited by: Comité de Comunicación de la Sociedad Española de Bioquímica Clínica y Patología Molecular, 2010 (ISBN: 84-89975-40-X).
The high prevalence (27.7%) of HIV-positive results in MTFs in certain populations reported in a recent meta-analysis makes it advisable to rule out HIV in these patients.28 The prevalence of positive HIV results in FTMs is low. Regular testing for HBV, HCV, and syphilis is recommended.
Current clinical guidelines1,17 do not state the need for a hypercoagulability study to be performed before the start of hormone therapy. However, the authors think that, given the increased risk of thromboembolic events in MTFs undergoing estrogen treatment as well as the easy access to this technique at hospitals, it is advisable for patients to grant their informed consent to it. By doing so, the potential risk increase is taken into consideration and patients may even benefit from prophylactic treatments if these are indicated before the start of CSHT.29,30
Contraindications to hormone therapyMTF-estrogens: thromboembolic disease, ischemic heart disease, stroke, active liver disease (transaminase levels greater than three times the upper normal limit), renal failure, severe hypertriglyceridemia, morbid obesity, poorly controlled diabetes, severe migraine, family history of breast cancer, prolactinoma.
FTM-androgens: active liver disease, renal failure, ischemic heart disease, severe hypertriglyceridemia, morbid obesity, poorly controlled diabetes mellitus.17,30,31
Eligibility criteriaThese are the minimum specific requirements that should be documented before the start of treatment. The following criteria should be met before starting hormone therapy1:
- 1.
A favorable report of the diagnostic evaluation by the MHP according to DSM-IV or ICD-10 criteria.
- 2.
Documentation of RLE for at least three months or completion of a psychotherapy period specified by the MHP of the team after initial evaluation (normally of at least three months).
- 3.
The patient's understanding of the different therapeutic options, including their benefits (real expectations) and health risks, the selection of the most adequate option, the patient's commitment to comply with the psychological and endocrine monitoring established.
Other criteria required by each gender dysphoria unit usually include age over 18 years or, failing this, age over 16 years and the legal guardian's consent, no contraindication to therapy after a review of the supplemental examinations requested, and a signature of informed consent.
All patients should be informed and counseled about the fertility options, before the start of hormone suppression if they are adolescents or before treatment with sex hormones of the desired sex in both adolescents and adults.2,17
Before hormone therapy is started, emphasis should be placed on smoking cessation, the performance of regular physical exercise, the adoption of a healthy diet, and the consumption of no more than 14 standard alcoholic drinks per week.
Hormone interventionThe two main objectives of hormone therapy are to decrease endogenous hormone levels and, thus, the secondary sexual characteristics of the biological (genetic) sex, and to replace these characteristics by those of the sex of identification, for which hormone therapy similar to that for hypogonadal patients is initially given.32,33 The total disappearance of these characteristics is not possible. In MTFs, skeletal development cannot be reversed if pubertal maturation has been completed, and neither complete facial hair eradication nor voice feminization is achieved. The desired breast development is not always achieved either. By contrast, feminizing genital surgery provides adequate cosmetic and functional results. In FTMs, male characteristics are easier to achieve. However, masculinizing genitoplasty does not currently provide for fully satisfactory cosmetic and functional results.2
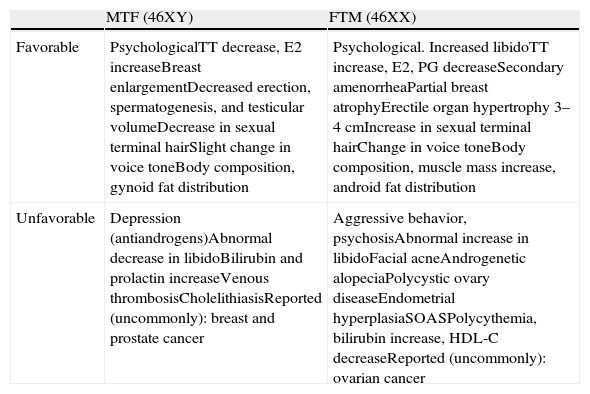
The physical and psychological changes expected with CSHT are shown in Table 3.17,29,34 The maximum effect on certain aspects may not occur until after two or three years of treatment. Heredity influences the response of target tissues to hormone therapy, with considerable between-subject variability, and its effect cannot be overcome by administering supraphysiological doses.
Favorable (desired) and unfavorable effects of cross-sex hormone treatment in adults with mismatch between gender identity and biological sex (29).
| MTF (46XY) | FTM (46XX) | |
| Favorable | PsychologicalTT decrease, E2 increaseBreast enlargementDecreased erection, spermatogenesis, and testicular volumeDecrease in sexual terminal hairSlight change in voice toneBody composition, gynoid fat distribution | Psychological. Increased libidoTT increase, E2, PG decreaseSecondary amenorrheaPartial breast atrophyErectile organ hypertrophy 3–4cmIncrease in sexual terminal hairChange in voice toneBody composition, muscle mass increase, android fat distribution |
| Unfavorable | Depression (antiandrogens)Abnormal decrease in libidoBilirubin and prolactin increaseVenous thrombosisCholelithiasisReported (uncommonly): breast and prostate cancer | Aggressive behavior, psychosisAbnormal increase in libidoFacial acneAndrogenetic alopeciaPolycystic ovary diseaseEndometrial hyperplasiaSOASPolycythemia, bilirubin increase, HDL-C decreaseReported (uncommonly): ovarian cancer |
SRS: sex reassignment surgery; SOAS: sleep obstructive apnea syndrome; MTF: male-to-female transsexual; FTM: female-to-male transsexual.
Most favorable effects start within 3 and 6 months of treatment start, and maximum effects are seen at 2–5 years.
These treatments should be prescribed and monitored by an endocrinologist experienced in the management of sex steroids. The selection of the hormone preparation and its release method and dosage should be guided by the principles of minimum risk for health and maximum efficacy.
Annex B includes information documents for patients currently being used in two national hospitals. It is important to explain verbally and in writing the changes that will occur in the body when hormone treatment is administered.
At this stage, the patient and the endocrinologist in charge will sign an informed consent stating not only the potential side effects of therapy, but also a commitment to comply with the established therapeutic deadlines and to assume indications and contraindications.2
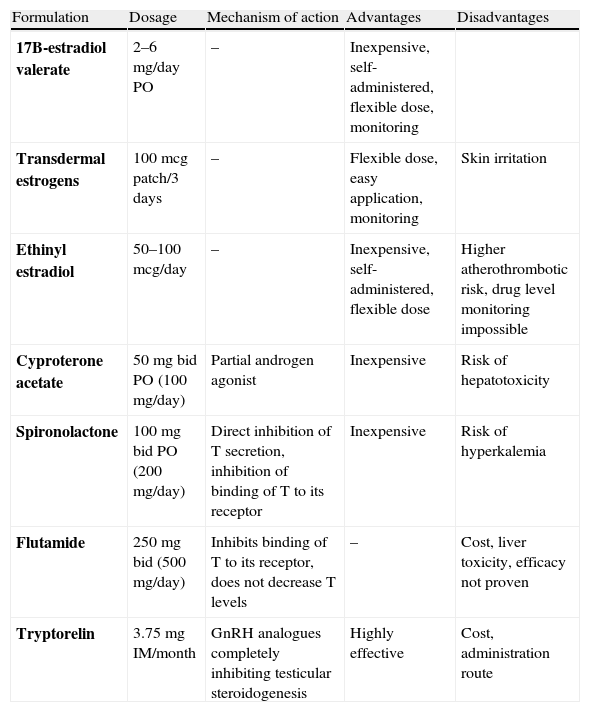
Cross-sex hormone treatment in MTF transsexualsIn transsexual women (MTFs), the treatment regimen is more complex than in FTMs. The most effective therapy is a combination of compounds that suppress endogenous production or the action of androgens and estrogens (Table 4).11,29,35 The aim is to eliminate sexual hair growth and induce breast formation and a female body fat distribution.
Clinical pharmacology and monitoring of cross-sex hormone treatment in MTFs (29).
| Formulation | Dosage | Mechanism of action | Advantages | Disadvantages |
| 17B-estradiol valerate | 2–6mg/day PO | – | Inexpensive, self-administered, flexible dose, monitoring | |
| Transdermal estrogens | 100mcg patch/3 days | – | Flexible dose, easy application, monitoring | Skin irritation |
| Ethinyl estradiol | 50–100mcg/day | – | Inexpensive, self-administered, flexible dose | Higher atherothrombotic risk, drug level monitoring impossible |
| Cyproterone acetate | 50mg bid PO (100mg/day) | Partial androgen agonist | Inexpensive | Risk of hepatotoxicity |
| Spironolactone | 100mg bid PO (200mg/day) | Direct inhibition of T secretion, inhibition of binding of T to its receptor | Inexpensive | Risk of hyperkalemia |
| Flutamide | 250mg bid (500mg/day) | Inhibits binding of T to its receptor, does not decrease T levels | – | Cost, liver toxicity, efficacy not proven |
| Tryptorelin | 3.75mg IM/month | GnRH analogues completely inhibiting testicular steroidogenesis | Highly effective | Cost, administration route |
Treatment goal. Estradiol levels should be maintained at the normal mean values in premenopausal women (200pg/mL) or in the upper limit of normal for the follicular phase in each reference laboratory, together with T levels in the female range (<0.8ng/mL).
Monitoring. E2 should be measured every three months in the first year, and every six months or annually thereafter. The time of E2 measurement will depend on the preparation used: 24h after the last oral dose of estrogen or 48h after transdermal estrogen application.
DHT: dihydrotestosterone; bid: twice daily; E2: estradiol; IM: intramuscular; w, week; T: testosterone; MTF: male-to-female transsexual; PO, by the oral route.
Estrogens should preferably be administered by the oral route (conjugated estrogens of estradiol valerate) or as transdermal preparations. The use of oral transdermal and non-synthetic estrogens allows for the monitoring of their levels. Estradiol levels should be maintained at the normal mean values for premenopausal women or in the upper limit of the normal follicular phase for each reference laboratory (estradiol 100pg/ml as measured by radioimmunoassay), together with total testosterone levels in the female limits (less than 0.5–0.8ng/ml by chemiluminescence immunoassay).
Current estrogen preparations of choice include estradiol valerate or transdermal estrogens. Oral 17β-estradiol valerate (2–6mg/day); transdermal estrogens (100μg/3–7 days). The use of transdermal estrogens may confer an advantage to older MTFs, who have an increased risk of thromboembolic disease.29
Conjugated horse estrogens are more difficult to monitor, and ethinyl estradiol should not be used because it is associated with a greater thromboembolic risk.17,29–31
The suppression of estrogen secretion or action is based on agents with antiandrogenic effects and gonadotropin-releasing hormone (GnRH) analogues.32 Cyproterone acetate (Androcur® 50mg) is a synthetic steroid of choice with a dual effect that blocks the androgen receptors and inhibits gonadotropin secretion.29 The standard dosage is 50mg twice daily, but treatment dose and duration should be adjusted, based on the androgen impregnation grade of each patient, and particularly on age at treatment start. Although uncommon, there are patients who achieve adequate feminization and decreased erections during follow-up without anti-androgen therapy, with estrogen treatment alone.2 Side effects usually include some weight increase and moderate water retention due to the drug itself and enhanced by associated estrogens. Hepatotoxicity and impaired nocturnal vision have been reported.33,34
Spironolactone (Aldactone® 100) is a diuretic with anti-androgen properties directly inhibiting testosterone secretion and binding to the androgen receptor32 at doses of 200mg/day. It is not routinely used. Other available anti-androgens include flutamide and finasteride, which prevent final testosterone action but do not decrease its levels. Their efficacy has not been shown.
Because of their high cost and lack of approval for this indication, GnRH analogues (Decapeptyl®) should be reserved for compassionate use for GID in adolescents, as they induce reversible chemical gonadectomy, and for patients refractory to anti-androgen preparations.2,32
Anti-androgen therapy is not required after gonadectomy.
The use of progestogens is controversial and initially not recommended, because they may increase thromboembolic risk, liver changes, high blood pressure, and mood changes, and enhance all the other potential negative effects of estrogen therapy. They may be used if estrogen dose reduction is required or in the event of estrogen intolerance.2 In such cases, medroxyprogesterone 2.5–10mg/day (as 5–10mg tablets) or noretisterone (as 5–10mg tablets) at the same dose is recommended.
Estrogen treatment should be continued after genital surgery at adequate doses to prevent clinical signs of hypogonadism and bone mass loss.1
Cross-sex hormone treatment in FTM transsexualsIn transsexual males, treatment is aimed at stopping menstruation and inducing virilization, including a sexual hair pattern, a male morphotype and hypertrophy of the erectile organ. Hormone treatment also results in increased muscle mass, decreased fat mass, and increased libido.
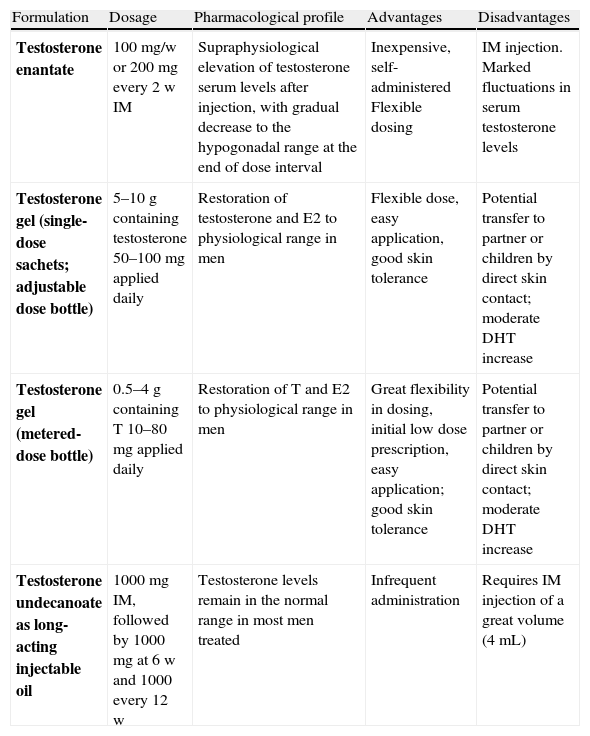
The most commonly used androgen preparations35 (Table 5) are testosterone esters administered either IM or as a topical gel (patches, although recommended, are not currently available on the Spanish market). The aim is to maintain total testosterone levels within the reference range for the male population (320–1000ng/dl). The appropriate time for measuring testosterone levels depends on the preparation (Table 5). The virilizing effect of androgens and their capacity to inhibit gonadotropins avoids the need for the mandatory use of anti-gonadotropin drugs to stop menstruation. Except in isolated cases, amenorrhea is achieved after 2–3 months of androgen treatment. Very rarely, GnRH analogues or progestogens should be used if the menstrual period persists.
Clinical pharmacology and monitoring of cross-sex hormone treatment in FTMs (29).
| Formulation | Dosage | Pharmacological profile | Advantages | Disadvantages |
| Testosterone enantate | 100mg/w or 200mg every 2 w IM | Supraphysiological elevation of testosterone serum levels after injection, with gradual decrease to the hypogonadal range at the end of dose interval | Inexpensive, self-administered Flexible dosing | IM injection. Marked fluctuations in serum testosterone levels |
| Testosterone gel (single-dose sachets; adjustable dose bottle) | 5–10g containing testosterone 50–100mg applied daily | Restoration of testosterone and E2 to physiological range in men | Flexible dose, easy application, good skin tolerance | Potential transfer to partner or children by direct skin contact; moderate DHT increase |
| Testosterone gel (metered-dose bottle) | 0.5–4g containing T 10–80mg applied daily | Restoration of T and E2 to physiological range in men | Great flexibility in dosing, initial low dose prescription, easy application; good skin tolerance | Potential transfer to partner or children by direct skin contact; moderate DHT increase |
| Testosterone undecanoate as long-acting injectable oil | 1000mg IM, followed by 1000mg at 6 w and 1000 every 12 w | Testosterone levels remain in the normal range in most men treated | Infrequent administration | Requires IM injection of a great volume (4mL) |
Monitoring. Total testosterone measurement every three months in the first year, and every six months or annually thereafter. The time of testosterone measurement will depend on the preparation used: for testosterone enantate IM, levels should be measured halfway between injections, for T gel at any time after receiving at least one week of treatment, and for long-acting testosterone undecanoate IM just before the next injection.
Treatment goal. To maintain testosterone levels of 3–10ng/mL (mean normal range for biological males according to the reference laboratory). Estradiol2 levels <50pg/mL (the upper normal limit for biological males according to the reference laboratory).
DHT: dihydrotestosterone; IM: intramuscular; w: week; FTM: female-to-male transsexual.
Androgen treatment should be continued after genital surgery at adequate doses to prevent hot flashes and bone mass loss.2
Adverse events and safety of cross-sex hormone treatmentA priori, CSHT has the same risks as replacement hormone therapy in hypogonadal patients, although the supraphysiological doses often required may be associated with a greater risk of complications.2,11,34 Patients with medical problems or a high risk of cardiovascular disease are more likely to experience serious or fatal effects from treatment with hormones of the opposite sex. The risks and side effects of hormone treatment may increase due to smoking, obesity, old age, heart disease, hypertension, abnormal coagulation, malignancies, or some endocrine abnormalities. Patient and physician should discuss the benefit–risk balance. Table 3 shows the potential adverse events.
Bone mineral density. Adequate hormone treatment dosage is important in order to maintain bone mass in transsexual people. There is little information in the literature about the risk of fracture in these patients, but the discontinuation of supervised treatment after gonadectomy is a risk factor for osteoporosis. In FTMs, the protective effect of testosterone appears to be mediated by peripheral conversion to estradiol, while in MTFs exogenous estrogens preserve bone mineral density.36,37
Cardiovascular disease. Little information is available in the literature about the mid and long-term impact of CSHT on cardiovascular disease.11 Some published observations report that androgen deprivation, combined with the estrogen environment, in MTFs is associated with greater harmful effects than the induction of an androgen environment in FTMs.9 However, the UTGI of Andalusia has reported an increasing incidence of metabolic syndrome over time, particularly in the FTM group.38 In order to decrease the risk of metabolic syndrome and cardiovascular disease, healthy hygiene and dietary measures should be promoted in these patients.
Risk of cancer. There are few reported cases of hormone-dependent cancer in patients with GID. However, the chance of cancer occurrence increases with the duration of exposure to cross-sex treatment and age.33,39 Few cases of breast cancer in MTFs have been reported in the literature. General population studies suggest that estrogen therapy does not increase the risk of breast cancer in the mid term (20–30 years).40,41 However, regular physical examinations should be performed by both patients and clinicians. Prostate cancer is uncommon before 40 years of age, and although MTFs do not appear to have a greater risk of suffering prostatic disease, the isolated cases reported in the literature of benign prostatic hypertrophy and three cases of prostate cancer in MTFs in whom therapy was started after 50 years of age make PSA measurement mandatory. Based on PSA levels, a subsequent digital rectal examination should be considered in elderly MTFs with late or intermittent CSHT.17 When hysterectomy is delayed in FTMs, there is a potential risk of endometrial cancer that would justify annual gynecological examination,33,39,40 a suggestion that is not always welcomed by patients.
Clinical and laboratory follow-upEndocrine follow-up will be more comprehensive during the first year of hormone therapy, and may be done at longer intervals after SRS. Table 2 shows the standard monitoring for these patients. Patients are initially assessed every 3–4 months during the first year and every six months thereafter for the rest of their lives. Weight, blood pressure, complete blood count, kidney and liver function, glucose metabolism, uric acid, and lipid profile should be monitored. Regular monitoring (every 2–3 years) of bone mineral density is required after gonadectomy performed to MTFs at genitoplasty or after hystero-oophorectomy in FTMs. The medical treatment phase is also an appropriate time for assessing patient satisfaction (or otherwise) with the physical changes experienced. This is a mandatory requirement in order to confirm the transsexual condition.2 The physiognomic and functional changes are fully or partially reversible in the early stages, but not when treatment has been administered for a long time.
Male-to-female transsexualThe objective is to maintain estrogen and androgen levels within the physiological range in biological women, in order to prevent the occurrence of thromboembolic events, liver dysfunction, or high blood pressure. Up to 20% of transsexual women show increased prolactin levels, whether or not associated with pituitary gland enlargement,42,43 due to chronic stimulation of lactotroph cells by estrogen therapy or to direct interference with prolactin inhibitory factor.44,45 Since the symptoms associated with this elevation are not assessable in MTFs (hypogonadism, gynecomastia), plasma level monitoring is essential. Estrogen dose reduction or discontinuation is usually sufficient to resolve the process.
As previously noted, limited evidence is available concerning the potential cardiovascular benefits or risks associated with estrogen therapy in MTFs.46 The favorable changes in lipid profile, including HDL cholesterol increase and LDL cholesterol decrease, appear to be counteracted by weight and blood pressure increases and changes in insulin homeostasis.38,47 Continuous cardiovascular risk assessment is therefore required in these patients.
In MTFs on estrogen therapy, it is suggested that the clinical guidelines for prostate disease and cancer recommended for biological men be followed, as well as the guidelines for breast cancer screening in biological women.17,33,34
Female-to-male transsexualThe objective is to maintain testosterone within the physiological range in biological men, avoiding the occurrence of adverse events associated with chronic androgen therapy,35 mainly including erythrocytosis, liver dysfunction, hypertension, excess weight gain, lipid changes, severe acne, and psychological changes.17,30,34,46 Before gonadectomy, LH levels may serve as an indicator of the suitability of sex steroid administration for preserving bone mass.17,36,37
There is no conclusive evidence of a lower or greater cardiovascular risk associated with androgen therapy at physiological doses in FTMs.48 General population data, showing an inverse relationship between endogenous testosterone levels and mortality,49,50 do not appear to be comparable to changes induced by hyperandrogenization in these patients. Testosterone influences body fat distribution mediated by the expression of specific sex steroid receptors in adipose tissue and by the local metabolism of steroid hormones.51 At cell levels, androgens act upon adipocytes to stimulate lipolysis, reducing fat deposits. However, testosterone administration to FTMs usually leads to a more atherogenic lipid profile, with decreased HDL cholesterol and increased triglyceride levels.2,52 The impact on insulin homeostasis in FTMs is controversial.38,47 Androgen-associated muscle mass increase may have beneficial effects because skeletal muscle is the main glucose uptake site under insulin stimulation conditions, taking up to 75% of available glucose in the postprandial period.52
Although additional studies assessing cardiovascular risk in FTMs are needed, these data justify the need for the continued evaluation of these risk factors in these patients.
The assessment of uric acid metabolism is also recommended because it experiences significant changes in both FTMs and MTFs with CSHT.53
FTMs require annual gynecological examination until hysterectomy and dual adnexectomy because of the potential risk of neoplasm.
In both patient groups, after at least two years of hormone treatment (to achieve the best tissue response to estrogens or androgens) and verification of adherence to the psychological and endocrinological follow-up protocol, patients may request a change in their registry documentation according to the recent Act 3/2007, of March 15.
The maximum age for the maintenance of CSHT is not well defined. No series of elderly transsexual people on follow-up have been reported. Strict prospective follow-up with monitoring for cardiovascular risk factors and sex steroid-dependent tumors, in addition to bone mass assessment, is recommended.
Management of gender dysphoria in children and adolescentsAs in adults, the diagnostic assessment of the sexual identity and mental health of children and adolescents is essential. Sex therapy and psychotherapy is aimed at resolving any existing comorbidity and at decreasing the discomfort experienced by patients related to their sexual identity problems. Conservative management is required because sexual identity may unexpectedly change in this age group,6,54–57 and no direct influence should therefore be exerted on gender role, nor should hormone treatment be started in prepubertal children. Their experience of the initial changes related to spontaneous puberty is vital, because their interpretation of these early physical changes has diagnostic value.
The eligibility criteria for starting any hormone intervention in adolescents are superimposable on those of adults, but there are additional requirements, including the achievement of the Tanner 2 stage of pubertal development and the availability of adequate psychological and social support. This support can influence the outcome of family psychotherapy and so guide parental decision making.
If continued dysphoria is confirmed, early medical intervention to suppress the gonadal axis in the early pubertal development stages in patients with GID may prevent psychological damage associated with the development of secondary sexual characteristics of their biological sex, leading to more adequate psychosocial adaptation and improved physical results.17 This is a reversible intervention, which allows for pubertal development of the biological sex in the future, if needed.
Such early intervention requires close follow-up by the endocrinologist. No treatment can be recommended if the Tanner 2 stage has not been reached and, if prescribed, GnRH analogues (Decapeptyl 11.25mg® IM/12 weeks) are used. Sexual characteristics in early pubertal stages will regress, while treatment started at more advanced stages will stop the process, leading to partial breast atrophy and the absence of menses in girls and to arrested virilization and decreased testicular and penile volume in boys.58 Monitoring of gonadotropin and sex steroid levels is required to confirm the adequate suppression of the gonadal axis and to shorten the therapeutic interval if needed. As regards potential adverse events, although the initial arrest of bone mineralization occurs, there are no conclusive data concerning the potential impact of this induction of “delayed puberty” on adult bone mineral density in these patients.
Other alternatives, such as the use of progestogens, antiestrogens, or antiandrogens, are less effective and cause more adverse events.17,58
Associated CSHT is started in adolescents from 16 years of age to induce puberty of the desired sex with progressive treatment titration superimposable on that for other hypogonadal adolescents:
MTF transsexuals: 17β estradiol 5mcg/kg/day with six-monthly increase for two years up to the adult dose (2mg/day).
FTMs: testosterone 25mg/m2/every two weeks with a six-monthly increase for two years up to the adult dose (Testogel® 50mg/day topically, Reandron® 1g IM every 12 weeks).
The current trend is long-term use of GnRH analogues from Tanner stage 2 to 18 years of age. Progressive estrogen or androgen doses are added to GnRH analogues at 16 years of age, and the sex reassignment process directly continues with genitoplasty when the age of majority is reached and after at least 1–2 years of CSHT.59
Other therapiesSexual hair removalSex reassignment treatment, either hormonal or surgical, results in a significant reduction of sexual hair growth, although this varies depending on the individual. There are currently only two potentially permanent hair removal methods, electrical epilation and laser. Electrical epilation (individual destruction of each follicle by thermal or electrolytic energy) is slow and cumbersome and requires repeated treatment for months or years. Although this method provides good results in experienced hands, it is only adequate for limited areas such as the upper lip. Laser epilation (light energy absorption by melanin causing destruction of hair follicles) is more rapid because it allows for treating several follicles at a time and is more effective for dark hair. However, it is more expensive and also requires several sessions, and permanent results are not guaranteed.
Speech and language therapyThis is sometimes needed because of the limited effect on voice changes in MTFs and the variable response in FTMs. It may be provided by a speech therapist individually or as group therapy.
Sex reassignment surgeryFor most patients with GID, genital surgical reassignment is a necessary step to achieving a fully satisfactory life in the sex role with which they identify. Surgical procedures have evolved in recent years, and genitoplasty with preservation of neurological sensitivity is currently the standard surgery. Each case should be individually assessed, which requires patient re-evaluation close to surgery by a member of the psychology/psychiatry team familiar with the case, and should also be discussed in a clinical session by all members of the unit (endocrinologists, mental health professionals, and surgeons involved in the procedures, urologists, gynecologists, and plastic surgeons).
Readiness criteria: these are specific criteria on which are based the clinical assessment of whether or not to continue with the treatment sequence (in this case, to pass from endocrinology to surgery).1,2
- 1.
The patient has increased consolidation of his/her gender identity during RLE or psychotherapy.
- 2.
The patient has made progress in the control of his/her identity problems leading to an improvement or continuity in his/her mental stability.
- 3.
Hormones are responsibly taken.
Criteria for surgery include:
- -
Age over 18 years; a successful RLE, with responsible participation in psychotherapy if needed, for at least 12 months.
- -
Supervised hormone therapy for at least 12 months.
- -
Understanding of the type of surgery, its irreversibility, hospitalization time, potential complications and rehabilitation, and a signature of informed consent.
- -
Approval by the multidisciplinary committee.
If hormone therapy is contraindicated, direct surgery may be recommended.
Genital surgery for MTFs. This consists of phallectomy, gonadectomy, and the formation of a neovagina from penile skin, using scrotal skin to create labia majora, preserving the neurovascular bundle of glans as tissue to create a clitoris.60
In MTFs, estrogen treatment should be discontinued between two and three weeks before surgery because of the risk of venous thrombosis, and restarted once the patient starts ambulation.
Genital surgery for FTMs. Genitoplastic surgery is less satisfactory. The cosmetic appearance of a neo-penis may eventually be adequate, but multiple procedures performed by experienced surgeons are required, and penile erection may only be achieved in a few cases using prosthetic systems. Hysterectomy plus dual adnexectomy should be performed no longer than 2–3 years after treatment start, because the long-term use of androgens has been associated with the occurrence of ovarian tumors.61 Today, surgery is usually performed using a laparoscopic approach. Masculinizing genitoplasty should not be performed before 2–3 years of androgen treatment in order to achieve the greatest hypertrophy of the erectile organ on which metoidioplasty will subsequently be performed, unless the patient and surgeon of the team prefer other surgical procedures.2
Breast surgery. The maximum estrogen effect on the breast gland is seen in MTFs after at least two years of estrogen treatment, and augmentation mammoplasty should therefore not be considered until this time. Breast surgery may be performed before or after genital surgery. In MTFs, however, mastectomy should be indicated at an earlier time once hormone therapy and RLE have started (6–12 months after these).
Circuit of services provision and patient referralEach unit should establish its patient referral model. Patients are usually referred by primary care physicians or mental health professionals from the healthcare area, but the demand for information or direct care by patients themselves is not uncommon. The situation also depends on whether the team is a reference center in the healthcare area or even on whether the unit accepts cases referred from other autonomous regions.
There are protocols where the case is jointly assessed at the first visit by a team of psychologists/psychiatrists and endocrinologists, while in others mental health professionals from the unit see the patients first. In other protocols, a coordinator determines the sequence of appointments and medical treatment.2,3,8
Research projectsIt would be convenient if national research projects could be undertaken where the different specialized units work in concert to advance their understanding of this clinical condition and to improve the care and quality of life of these patients. Some units have already implemented joint assessment and data collection protocols which have resulted in several recent publications.62–65
Conflicts of interestThe authors state that they have no conflicts of interest.
Albero, Ramón (Aragon), Almaraz, María Cruz (Andalusia), Álvarez-Diz José Antonio (Asturias), Audí, Laura (Barcelona), Becerra, Antonio (Madrid), Castaño, Luis (Basque Country), Esteva, Isabel (Andalusia), Fernández Sánchez-Barbudo, Miguel (Canary Islands), Gómez-Balaguer, Marcelino (Valencian Region), Gómez-Gil, Esther (Catalonia), Hurtado Felipe (Valencian Region), López-Siguero, Juan Pedro (Andalusia), Martínez-Tudela, Juana (Andalusia), Moreno-Pérez, Óscar (Valencian Region), Sanisidro, Carmen (Aragon), Toni, Marta (Navarre), Vázquez-San Miguel, Federico (Basque Country), and Vidales, Angelines (Basque Country).
Department of Endocrinology and Nutrition
Unit of Transsexualism and Gender Identity
Hospital Civil, Pabellón 7, 2.ª planta (Pabellón C, HRU Carlos Haya). Malaga
The following effects of treatment occur gradually and not always in the same form in each person. Action usually starts to be noticed from 2 to 4 months of treatment, and some effects are irreversible after 6–12 months of treatment. High estrogen doses should not be used, because lower doses achieve similar mid and long-term results and also cause fewer side effects when adequately used.
Results may be less evident if testicular excision surgery has not been performed.
- 1.
On sexual activity: erectile capacity decreases until it almost completely disappears. Because of decreased sperm secretion, ejaculatory orgasms are less intense.
Sexual satisfaction is variable and subjective, with great variations depending on each person.
Tests and prostate become atrophic.
- 2.
Fertility decreases due to decreased sperm production. This may sometimes be reversible upon treatment discontinuation, but permanent sterility may occur after six months of estrogen use.
- 3.
Increased breast size. Growth is not always symmetrical in both glands, and a specifically desired size cannot be chosen by increasing estrogen dose.
- 4.
Fat is redistributed, increasing in the hip area and also changing in the face.
- 5.
Body hair decreases (facial hair does not always completely disappear, but becomes less dense and dark).
- 6.
The voice experiences little change because the adult larynx is poorly sensitive to these hormones.
- 7.
Changes in character, with increased emotionality, although this greatly varies depending on the psychological characteristics of each person.
Other (uncommon) potential side effects include occasional fluid retention, liver function impairment in approximately 4 out of every 100 patients, gallbladder stone (1% of patients), and thrombophlebitis or pulmonary thromboembolism in very exceptional cases.
If testis removal is not requested, a specialist must perform regular examinations, because degenerative lesions may very occasionally occur.
The following effects of treatment occur gradually and not always in the same form in each person. Treatment action usually starts to be noticed from the first androgen doses, and the effects are virtually irreversible almost from the start. High androgen doses should not be used, because lower doses achieve similar mid and long-term results and also cause fewer side effects when adequately used.
- 1.
Fertility decreases, menstrual cycles disappear.
- 2.
Sexual activity increases, pain may occasionally occur in the erectile organ (clitoris), which increases in size by several cm.
- 3.
Facial and body hair increases and becomes thicker and darker.
Acne may appear.
- 4.
Muscle mass increases. Male fat distribution is seen.
- 5.
The voice may become deeper, but does not always achieve a classical male tone.
- 6.
Changes in character, with increased aggressiveness, although this greatly varies depending on the psychological characteristics of each person.
- 7.
The breast glands scarcely decrease in size, but they may become softer.
Some potential side effects or metabolic changes you should be aware of include occasional fluid retention, headache, increase in blood pressure levels, increase in blood glucose levels, and increase in cholesterol levels.
Our unit will give you information about all of these.
The maximum physical effects of hormones may not occur until continuous treatment has been administered for two years. Heredity influences the response of tissues to hormones, and this influence cannot be overcome by administering higher doses. The effects actually achieved vary from patient to patient.
Biological males treated with estrogens may expect treatment to effectively result in some breast growth, some body fat redistribution to a more female form, a decreased strength in the upper part of the body, skin softening, decreased body hair, decreased baldness, decreased fertility and testicular size, and less frequent and firm erections. Most of these changes are reversible, but breast enlargement will not be completely reversed upon treatment discontinuation.
Biological women treated with testosterone may expect the following permanent changes: a deeper voice, growth of the clitoris, moderate breast atrophy, increased face and body hair, and typical male baldness. Reversible changes include increased strength in the upper part of the body, weight increase, increased social and sexual interest and sexual excitement, and decrease in hip fat.
Patients with medical problems or a trend to cardiovascular disease are more likely to experience serious or fatal effects of treatment with hormones of the opposite sex. The risks and side effects of hormone treatment may increase because of smoking, obesity, old age, heart disease, hypertension, abnormal coagulation, malignancies, and some endocrine abnormalities. Thus, some patients may not tolerate the use of hormones of the opposite sex. In addition, hormones may have both benefits and risks for health. Patient and physician should discuss the benefit-risk balance.
In biological males treated with estrogens and progestogens, the side effects may include an increased trend to coagulation (venous thrombosis with a risk of fatal pulmonary embolism), the development of benign pituitary prolactinoma, infertility, weight increase, emotional instability, liver disease, gallbladder stone formation, drowsiness, high blood pressure, and diabetes mellitus.
In women treated with testosterone, the side effects may include infertility, acne, emotional instability, increased sexual desire, change in lipid profile to a male pattern (which increases the risk of cardiovascular disease), and a predisposition to develop benign and malignant liver tumors and liver malfunction.
The reason why you are being seen at this department is a clinical condition called gender dysphoria, which is also known as transsexualism (ICD F64.0).
This is a problem of which the cause is not well known, but in which a dissociation exists between your genetic sex at birth and your current psychological and social identity.
Other medical departments are also providing you with care in order to comprehensively manage this identity conflict. Joint assessment and monitoring by the endocrinology and psychology departments will be performed for at least six months before any hormone treatment is started in order to confirm the diagnosis and to rule out any contraindications of such treatment.
After this initial stage, endocrinology (UTGI) will perform a clinical history and a number of regular examinations to assess your physical status. Examinations may include a chromosome study (karyotype), routine blood and urine tests, hormone tests, and genetic tests that will be carried out as part of a research project to study their contribution to your diagnosis or your response to hormone treatment during follow-up.
After a diagnosis has been made, you will receive hormone treatment to suppress your current hormones and use other hormones that induce changes in your body according to the sex advised by your clinical and psychological state:
- •
MALE-TO-FEMALE TRANSSEXUALISM
- •
FEMALE-TO-MALE TRANSSEXUALISM
You will gradually notice the effects occurring in your body. The time required for the final results cannot be exactly foretold, because these results sometimes do not exactly agree with your expectations. Each step will be explained to you, but you should be aware that a great part of these changes are irreversible once treatment has been started.
After the psychological and hormonal treatment phase, you will be offered, if you so desire, the possibility of surgical treatment to remove your sexual organs and to have them reconstructed in accordance with your sexual identity.
CONSENT:
I,.................................., state that I have read this document and have received a satisfactory explanation of the nature and purposes of the project, as well as the most common and/or significant complications of the treatment. The phases of the project have also been explained to me, and I accept these times and the risk of potential unfavorable consequences that are not due to professional malpractice.
Signature: Signature of physician: Date:
http://www.san.gva.es/comun/ciud/docs/pdf/Castellano_201109_4.pdf
http://www.san.gva.es/comun/ciud/docs/pdf/Castellano_201109_5.pdf