Increased adiposity has been associated to worse metabolic profile, cardiovascular disease, and mortality. There are two main adipose tissue depots in the body, subcutaneous and visceral adipose tissue, which differ in anatomical location. A large body of evidence has shown the metabolic activity of adipose tissue; lipectomy and/or liposuction therefore appear to be alternatives for improving metabolic profile through rapid loss of adipose tissue. However, surgical removal of adipose tissue may be detrimental for metabolism, because subcutaneous adipose tissue has not been associated to metabolic disorders such as insulin resistance and type 2 diabetes mellitus. In addition, animal studies have shown a compensatory growth of adipose tissue in response to lipectomy. This review summarizes the implications of obesity-induced metabolic dysfunction, its relationship with the different adipose tissue depots, and the effects of lipectomy on cardiometabolic risk factors.
El aumento de la adiposidad se ha asociado con un peor perfil metabólico, enfermedad cardiovascular y mortalidad. Hay 2 depósitos principales de tejido adiposo en el cuerpo, el tejido adiposo subcutáneo y el tejido adiposo visceral, y difieren en la localización anatómica. Un gran cuerpo de evidencia ha demostrado la actividad metabólica del tejido adiposo; por lo tanto, la lipectomía y/o la liposucción parecen ser alternativas para mejorar el perfil metabólico a través de la pérdida rápida de tejido adiposo. Sin embargo, la extirpación quirúrgica del tejido adiposo podría ser perjudicial para el metabolismo, ya que el tejido adiposo subcutáneo no ha sido asociado con trastornos metabólicos, tales como resistencia a la insulina y diabetes mellitus tipo 2. Además, estudios en animales han demostrado un crecimiento compensatorio del tejido adiposo en respuesta a la lipectomía. Esta revisión resume las implicaciones de la disfunción metabólica inducida por la obesidad, su relación con los diferentes depósitos de tejido adiposo y los efectos de la lipectomía sobre los factores de riesgo cardiometabólico.
Obesity is one of the principal modifiable risk factors for an unhealthy status. It plays an important role on the global disease burden and is associated to both all-cause and cardiovascular mortality.1 Body mass index (BMI) is the universal method used for characterizing the excess of body mass. It is independent of age and has the same cutoff points for both genders; however, the World Health Organization (WHO) specialists committees have proposed distinct cutoff values for BMI according to ethnics, considering their differences in body composition and body fat distribution2 (Table 1). Nowadays, the traditional cutoff points for the general population still prevail and it is estimated that by 2030 2.16 billion people worldwide will fit into the “overweight” definition, and 1.12 billion will be obese, according to the BMI.3
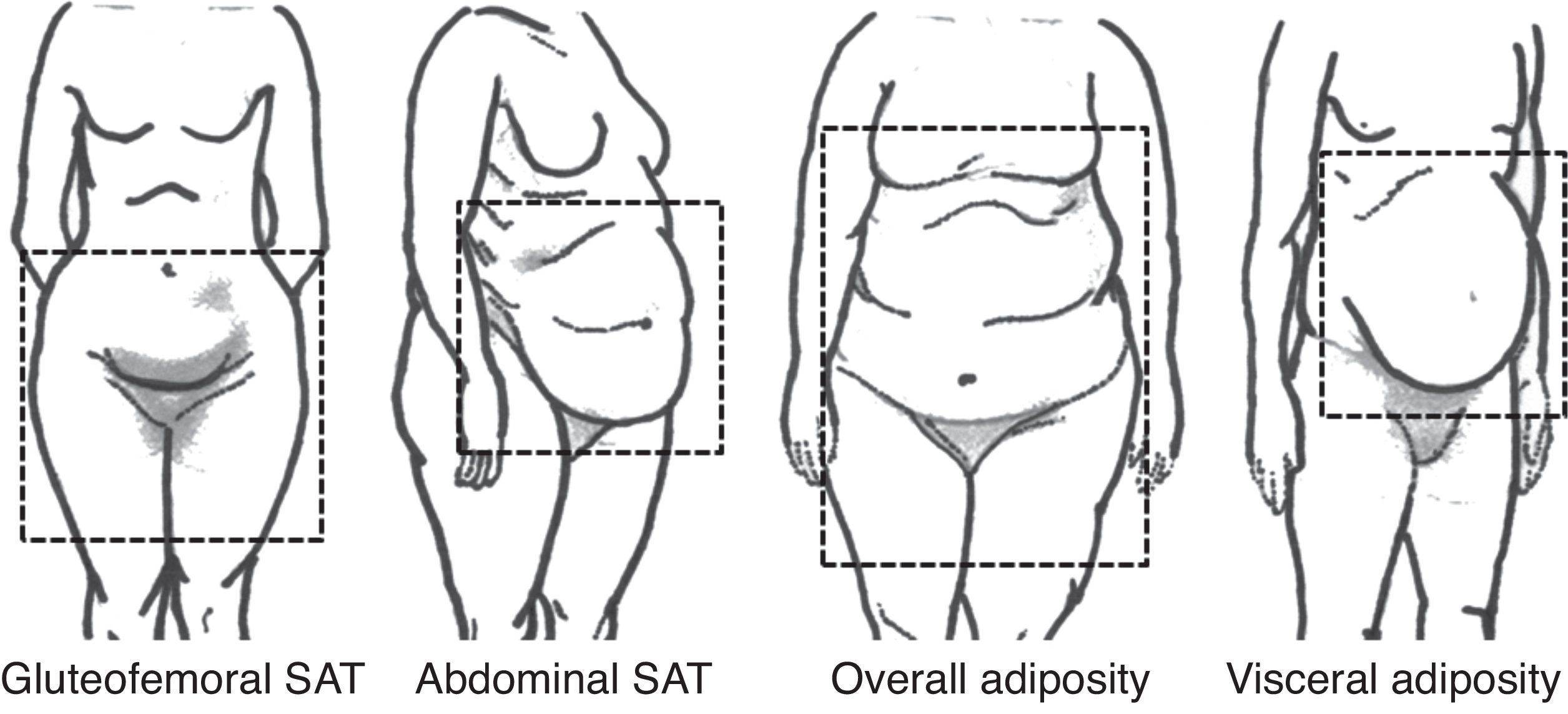
Proposed classification of total body adipose tissue.7
| 1. Total adipose tissue |
| 2. Subcutaneous adipose tissue |
| 2.1 Superficial subcutaneous adipose tissue |
| 2.2 Deep subcutaneous adipose tissue |
| 3. Internal adipose tissue |
| 3.1 Visceral adipose tissue |
| 3.1.1 Intrathoracic adipose tissue |
| 3.1.1.1 Intrapericardial |
| 3.1.1.2 Extrapericardial |
| 3.1.2 Intraabdominopelvic adipose tissue |
| 3.1.2.1 Intraperitoneal |
| 3.1.2.2 Extraperitoneal |
| 3.1.2.2.1 Intraabdominal |
| 3.1.2.2.2 Preperitoneal |
| 3.1.2.2.3 Retroperitoneal |
| 3.1.2.3 Intrapelvic |
| 3.2 Nonvisceral adipose tissue |
| 3.2.1 Intramuscular adipose tissue |
| 3.2.2 Perimuscular adipose tissue |
| 3.2.2.1 Intermuscular adipose tissue |
| 3.2.2.2 Paraosseal adipose tissue |
| 3.3 Other nonvisceral adipose tissue |
Adapted from Shen et al.7
Adipose tissue has distinct morphological and histological characteristics, and executes a number of activities. In mammals, three types of adipose cells are present: brown, white and beige.
Brown adipose tissue (BAT), full of mitochondria and specialized in heat production, exerts its thermogenic function mainly in the first years of life. Posteriorly, it transforms into white adipose tissue (WAT). However, BAT increased activity has been demonstrated in adults exposed to low temperatures; interestingly, this thermogenic effect is inversely related to age – older subjects have diminished expression of uncoupling protein 1 (UCP1) – and BMI.4,5
Beige adipose tissue, still under investigation, has less well-known functions and it is believed that it originates from white adipocytes trans-differentiation within the same cellular lineage (Pax7/myf5-). In animal models, it was demonstrated that these cells, initially, have a reduced UCP1 expression but, if stimulated, attain the capacity to increase the activity of these thermogenesis-related proteins. Genetically, they have an intermediate behavior between brown and white fat tissues; thus, these cells can store the excess of energy – in form of lipids – in situations where energetic balance is positive, and dissipates energy to produce heat in cases of thermogenesis stimulation.6
WAT is the most abundant adipose tissue in human organism and is responsible for lipid storage – in the form of triglycerides, mechanical protection and thermic isolation. However, interest lies on its capacity of secrete a number of substances with important roles in cardiovascular risk and protection, the adipokines. There is no consensus about WAT compartments nomenclature. Based on anatomy and functional properties, Shen et al. proposed a classification for body fat with emphasis on internal compartments, detected by image exams (Table 1).7 On the other hand, Foster et al. propose a visual classification, according to body fat distribution (Fig. 1).8
Subcutaneous white adipose tissue (SAT) has different distribution according to gender. In men there is increased accumulation in the trunk compared to limbs, with a decreased rate and a more balanced distribution after the age of 50; in women, accumulation of SAT is similar in abdomen and limbs until adulthood, when there is an increase of speed and amount of SAT accumulated in the abdomen.9,10
Visceral adipose tissue (VAT), located between the walls of abdominal cavity, is generally more prevalent in men than in women. There seems to be a proportional accumulation of VAT according to total adiposity in men, but not in women, who tend to accumulate fat in the abdominal cavity after reaching a certain level of total adiposity. Noteworthy is the fact that women also tend to increase the rate of VAT accumulation after menopause.10
Fat tissue distribution also modifies with aging. In the elderly, while total body fat may remain stable or even reduced, abdominal SAT is redistributed to VAT. During aging, the capacity of replication and differentiation of pre-adipocytes in subcutaneous mature adipocytes during adipogenesis is diminished, creating cells not fully capable of store fatty acids; thus, these cells can expand to visceral compartments.5,10
Metabolic implicationsAdipose tissue is directly controlled by the autonomous nervous system, where sympathetic fibers relate to catabolic activities (lipolysis) mediated by catecholamines, beta-adrenergic receptors (β1, 2, 3) and the hormone-sensitive lipase (HSL) enzyme.11 On the other hand, the parasympathetic fibers modulate anabolic effects in adipocytes (lipogenesis), promoting insulin-mediated fatty acids uptake.12 It is known, however, that exposure to cold may also indirectly modulate adipose tissue activity (brown and white) through macrophages recruitment and interleukin-4 (IL-4) activation, increasing secretion of lipolysis-inducting catecholamines in WAT and expression of thermogenic genes in BAT.13
Excessive nutrient intake (besides a decrease in energy expenditure) can lead to a number of responses in different cells (i.e. endothelial, hepatocytes, adipocytes) and cause a series of metabolic dysfunctions. Specifically regarding endothelial cells, excess of nutrients may induce inflammation and impair nitric oxide (NO) – a potent vasodilator – production and release. Similarly, alterations in adipose tissue perfusion can be present.14
In cases of chronic energy imbalance, there is excessive triglyceride accumulation in adipocytes which is translated in augmented intra-cellular fat content, leading to adipocyte growth (hypertrophy) and multiplication (hyperplasia). Hypertrophy, especially if accompanied by vascular supply insufficiency, leads to adipocytes hypoxia and, consequently, production of reactive oxygen species (ROS); this may cause oxidative stress, damaging cell structures and triggering an inflammatory response.14,15
Hypertrophied adipocytes are more fragile and susceptible to rupture, even when exposed to ordinary physical forces; cells in the abdominal cavity are subjected to sudden variations of intra-abdominal pressure (i.e. during cough and/or exercise). Obese individuals have elevated intra-abdominal pressure, putting visceral adipocytes under a higher mechanical stress compared to those in SAT. Macrophage accumulation [major sources of cytokines, like TNF-α and IL-6, which modulate hepatic production of C-reactive protein (CRP)] in the adipose tissue occurs mainly around ruptures adipocytes, where inflammatory cells sequestrate residual lipid droplets. Adipose cells apoptosis starts a cascade of events that lead to a chronic inflammatory state (low grade inflammation) related to obesity complications.4,15
Factors that regulate adipocytes hypertrophy and hyperplasia are yet to be completely clarified; however, insulin and glucocorticoids in high levels seem to stimulate pre-adipocyte differentiation. Hypertrophied cells increase the production of insulin-like growth factor 1 (IGF1) that also stimulates hyperplasia. Thus, as consequence of a number of different processes, production of adipokines is impaired in obese persons.14,15
Both subcutaneous and visceral adipose tissues have a differentiated behavior when it comes to adipokines. In VAT, there is predominance of β1, 2 and 3 adrenergic receptors compared to α2 ones, which make it extremely sensitive to catecholamine-induced lipolysis (with consequent increase in free fatty acids release directly into portal circulation). There is also a minor effect in insulin signaling with a reduction in anti-lipolytic effects, due to a decrease in insulin receptors substrate 1 (IRS-1). Additionally, there is augmented expression of 11-Beta hydroxysteroid dehydrogenase type 1 (11β-HSDH1), responsible for conversion of cortisone into cortisol, enhancing the response to glucocorticoids and, consequently, fat accumulation. Production of pro-inflammatory adipokines is greater in VAT. SAT, in turn, fundamentally regulates appetite, energy expenditure and fat deposits through increased production of leptin. Compared to VAT, lipolysis rates are nearly 50% inferior – gluteo-femoral compartments of subcutaneous fat have decreased response to catecholamines, lower density of adrenergic receptors and diminished HSL expression.14,15Table 2 presents some adipokines secreted by adipose tissue and their respective functions.
Adipokines produced and secreted by adipocyte.15
| Adipokine | Biologic effect |
|---|---|
| Adiponectin | Increases sensitivity to insulin; anti-inflammatory, anti-atherogenic |
| Adipsin | Activates complement alternative pathway |
| Angiotensinogen | Precursor of angiotensin; blood pressure regulation |
| Apelin | Control body energy stores; endothelial cell proliferation |
| ASP | Stimulates triglyceride synthesis in WAT |
| HGF | Stimulation of adipocytes differentiation and development |
| IGF1 | Stimulation of adipocytes proliferation and differentiation |
| Interleukin-10 | Anti-inflammatory; inhibits TNF-α production |
| Interleukin-6 | Pro-inflammatory; lipolysis; decreases sensitivity to insulin |
| Leptin | Signaling body energy stores |
| MCP-1 | Pro-inflammatory; insulin resistance; atherosclerosis |
| PAI-1 | Inhibits plasminogen activation (blocks fibrinolysis) |
| Resistin | Increases resistance to insulin |
| Sfrp5 | Anti-inflammatory; increases sensitivity to insulin |
| Tecidual factor | Starts coagulation cascade |
| TNF-α | Lipolysis; increases energy consumption; decreases sensitivity to insulin |
| VEGF | Stimulation of vascular proliferation (angiogenensis) in WAT |
| Visfatin | Insulin-like; produced mainly by VAT |
Adapted from Fonseca-Alaniz et al. WAT, white adipose tissue; VAT, visceral adipose tissue; ASP, acylation-stimulating protein; HGF, hepatocyte growth factor; IGF-1, insulin-like growth factor-1; MCP-1, monocyte chemotactic protein 1; PAI-1, plasminogen activator inhibitor-1; Sfrp5, secreted frizzled-related protein 5; TNF-α, tumor necrosis factor alpha; VEGF, vascular endothelial growth factor.
Some myokines (polypeptides secreted by muscles) are also produced by adipose tissue and have a duo effect: when there is adiposity excess, these proteins exert a pro-inflammatory role; on the other hand, after physical exercise, they have beneficial properties. Interleukin-6 is one of these adipomyokines; its effect in insulin resistance and lipogenesis is completely counteracted after exercise, increasing insulin sensitivity and lipid oxidation in striate muscle.16 Another peptide secreted by muscle after physical exercise, irisin (expressed by the fibronectin type III domain containing 5 – FNDC5 – gene) was recently discovered and is regulated by peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC1-α). Irisin activates thermogenesis in adipose tissue, stimulates beige adipose tissue and the “darkening” capacity of WAT in vivo and in vitro, promoting some metabolic functions and energy dissipation in thus cells through an increase in UCP1 expression.16,17 Recently, irisin was found to be secreted by adipocytes as well, mainly in subcutaneous tissue; in fact, endurance exercises acutely stimulate irisin secretion by VAT and SAT.17 Obese animals have an over-expression of this peptide, which suggests some level of resistance to its effects in obesity.
It is suggested that altered fatty acids and glucose metabolism may not be a direct consequence of VAT activity, but a reflex of abdominal SAT lack o capacity to adapt and expand through hyperplasia. Morphological and physiological characteristics of SAT could be responsible for its cells multiplication capacity in order to accommodate the excess of circulating fatty acids (from the positive energy balance) and to build a protector energy deposit. However, in cases of tissue hypoxia, the excess of circulating triglycerides would automatically migrate to the intra-abdominal compartment and accumulate in organs like liver, heart and pancreas (i.e. ectopic fat deposition). Women have higher degrees of lipolysis and free fatty acids mobilization from VAT compared to men.18
Therefore, abdominal deposits of visceral and subcutaneous fat may have different characteristics and exert unequal roles in cardiometabolic profile. This hypothesis was evaluated in men and women from Framingham in whom abdominal SAT and VAT were detected by image exams and analyzed separately in relation to hypertension (HTN), type-2 diabetes mellitus (T2DM) and metabolic syndrome (MS). VAT was more strongly associated to all co-morbidities than abdominal SAT, especially in women.19 Similar results were seen in more than 2400 subjects from the Jackson Heart Study cohort, where visceral and subcutaneous fat compartments were associated to HTN, T2DM and MS, with a higher risk for those with increased VAT compartments.20
The INternational Study of Prediction of Intra-abdominal adiposity and its RElationships with cardioMEtabolic risk/Intra-Abdominal Adiposity (INSPIRE ME IAA) study, which allocated patients from 29 countries, also determined VAT contribution on T2DM. Higher levels of visceral obesity (VAT area >204.9cm2 in men and >167.3cm2 in women) detected by computed tomography scan were associated to higher risk for T2DM in both genders, while SAT was not related to diabetes in men and had an inverse relationship with the disease in women (20% risk reduction).21
There is also evidence of the association between VAT and cardiovascular disease, regardless of general obesity and abdominal SAT. Among Brazilian subjects in whom coronary angio-computed tomography scan was performed, a VAT area ≥145cm2 was associated to the presence of coronary artery disease; the same was not observed with conventional anthropometric measures (waist circumference, waist-hip ratio and BMI).22
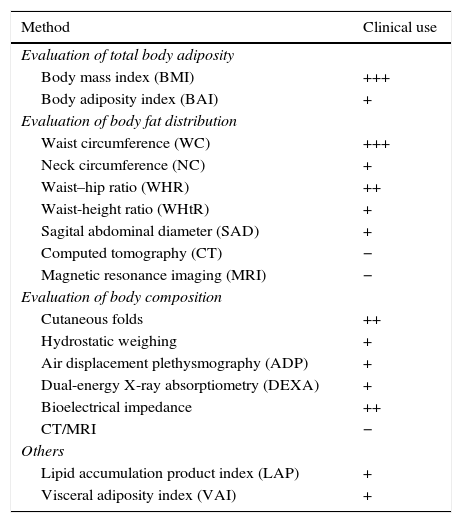
Adiposity excess can be evaluated by methods capable of detecting total body fat, body fat distribution and body composition (direct or indirect measurement of fat percentage). Table 3 shows some of these methods and their use in clinical practice.
Methods for body fat evaluation and use in clinical practice.23
| Method | Clinical use |
|---|---|
| Evaluation of total body adiposity | |
| Body mass index (BMI) | +++ |
| Body adiposity index (BAI) | + |
| Evaluation of body fat distribution | |
| Waist circumference (WC) | +++ |
| Neck circumference (NC) | + |
| Waist–hip ratio (WHR) | ++ |
| Waist-height ratio (WHtR) | + |
| Sagital abdominal diameter (SAD) | + |
| Computed tomography (CT) | − |
| Magnetic resonance imaging (MRI) | − |
| Evaluation of body composition | |
| Cutaneous folds | ++ |
| Hydrostatic weighing | + |
| Air displacement plethysmography (ADP) | + |
| Dual-energy X-ray absorptiometry (DEXA) | + |
| Bioelectrical impedance | ++ |
| CT/MRI | − |
| Others | |
| Lipid accumulation product index (LAP) | + |
| Visceral adiposity index (VAI) | + |
+++ widely accepted method; ++ accepted method; + unusual/rarely used method; − not recommended. Adapted from Cornier et al.
Considering the beneficial effects of SAT, the cardiometabolic safety of procedures in which part of this tissue is removed – such as lipectomy and liposuction – has been questioned. VAT remains intact during liposuction in contrast to SAT and it is concern that removal of these portions of WAT could result in substantial increase of VAT volume. Studies in animals showed that mice in a lipid-rich diet, and in which lipectomy was performed, had insulin resistance (IR), high triglyceride levels and hepatic steatosis 90 days after the procedure.24
In humans, results are controversial once many studies did not demonstrate harmful cardiometabolic effects of lipectomy neither showed benefits. Klein et al. demonstrated in obese women without glucose intolerance (BMI 35.1±2.4kg/m2) and with T2DM (BMI 39.9±5.6kg/m2) that after three months of SAT removal through lipectomy, despite the significant reduction of weight, general and abdominal adiposity and improvement in leptin levels, there was no improvement in insulin sensitivity. Moreover, no change was seen in fast plasma glucose, TNF-α, IL-6, adiponectin, CRP, lipid profile and blood pressure.25 Another study, conducted with eutrophic women who had small amounts of SAT removed, did not show changes in weight, insulin levels and insulin sensitivity by HOMA-IR 21 days after surgery; however, there was an improvement in lipid profile.26
A meta-analysis with 15 studies and 357 subjects showed that, after adjusting for post-surgery follow-up time (mean: 3 months) and BMI, only leptin and insulin serum levels were significantly related to the amount of aspirated fat; no association for CRP, IL-6, adiponectin, resistin, TNF-α, HOMA index, total, HDL and LDL cholesterols, triglycerides, blood pressure and free fatty acids. Based on the results, authors concluded that there is no evidence to support the hypothesis that subcutaneous fat removal reduces early cardiovascular or metabolic disease, its markers or its risk factors.27 One hypothesis is that the decrease in abdominal SAT, although substantial after liposuction, may have been insufficient enough to overcome the negative effects of the excess visceral fat on insulin sensitivity, cytokine production and lipid profile in individuals with excess of adiposity.24
Effects of lipectomy on body fat distribution have been evaluated. According to the “lipostatic” theory,28 in the long term, energy balance is regulated by a number of feedback systems that favor regulates body fat deposits. A sudden decrease/removal of adipose tissue (as in lipectomy) can activate feedback mechanisms that favor the recovery of this “eliminated” fat, by reducing energy expenditure and increasing food intake. In a number of species, when adipose tissue is surgically removed, this recovery happens in weeks to months and because of an increase in fat located in intact deposits – and not because of reaccumulation of aspirated deposits.24
In animal models, this compensatory ability is not uniform and depends on the place where adipose tissue was extracted from. Similarly, mode of compensation can be different according to the location of aspirated deposits: intra-abdominal fat tissue reaccumulates trough adipocytes hypertrophy while subcutaneous fat reccumulates through hyperplasia.24 Leptin seems to be the main inductor of compensatory responses and reaccumulation of fat mass; its levels are significantly reduced short after lipectomy and remain low until three months after surgery. This response is expected because SAT adipocytes are the primary source of leptin in humans. This hormone acts in the hypothalamus inhibiting food intake, increasing energy expenditure and balancing adiposity levels; in the periphery, its actions are increase in lipolysis and free fatty acids oxidation in striate muscle.29 Decreased levels of leptin after lipectomy could activate compensatory responses that enhance food intake and decrease energy expenditure, which would facilitate weight and fat regain.24 Further, lipectomy results in decreased norepinephrine turnover in non-excised adipose tissue pads, implying that decreased sympathetic tone may contribute to lipectomy-induced compensatory increases of fat mass by means of promoting lipid accretion through decreased basal lipolysis.8
In humans, a randomized trial with non-obese women (BMI 24.5±2kg/m2) in whom DEXA and cutaneous folds measurement (determination of body composition), and waist circumference and MRI (detection of abdominal obesity) were performed, evaluated the effects of removing small volumes of subcutaneous tissue by lipectomy (thighs and abdomen). Follow-up was performed in 6 weeks, 6 months and 1 year after the procedure. After 6 weeks, total body fat percentage was significantly reduced in those located to the “lipectomy” group, but this difference did not exist after 6 months. Reaccumulation of fat tissue was different according to the analyzed body region; after 1 year, gluteofemoral subcutaneous tissue in those women who performed surgery was unaltered, while abdominal fat percetage was significantly increased.30 Authors concluded that following suction lipectomy, body fat was restored and redistributed from the thigh to the abdomen.
ConclusionObesity is a chronic disease associated with higher risk for metabolic disorders; however, adipose tissue has different characteristics according to its location and morphology. Subcutaneous adipose tissue depots seem to be negatively correlated with cardiovascular risk factors while higher levels of visceral adipose tissue have been strongly associated with metabolic dysregulation. Fat manipulation such as lipectomy and liposuction have been utilized as alternatives for improvement of both body composition and cardiometabolic factors; however, the results of studies investigating the effects of liposuction on the metabolic profile are inconsistent and most reporting either no change or improvements in one or more cardiovascular risk factors. Besides, it is suggested that subcutaneous adipose tissue excision may lead to increased visceral accumulation. Thus, there is insufficient evidence to support the hypothesis that subcutaneous fat removal reduces early cardiovascular or metabolic disease, its markers or its risk factors.
Source(s) of supportNone.
Conflict of interestNone.