Resistance to thyroid hormone (RTH) is an uncommon syndrome in which there is a decreased sensitivity to thyroid hormones. It is characterized by elevated free T3 and T4 levels with normal or slightly increased TSH levels. RTH may coexist with autoimmune thyroid disease, and an association with Hashimoto thyroiditis has more frequently been documented.
We report the case of a female patient with RTH who developed hyperthyroidism due to Graves-Basedow disease (GBD) and methimazole-induced toxic hepatitis.
This was a 39-year-old woman referred for high TSH and free T4 levels. No symptoms suggesting thyroid dysfunction were found in a problem-oriented medical history. The patient did not complain of headache or visual changes. She was not aware of any personal history of interest, and physical examination revealed 49.7kg of weight, 1.48m of height, a heart rate of 87 beats per minute, normal blood pressure, and palpable grade Ib-II goiter. Laboratory test results were as follows: free T4 2.81ng/dL (0.9–1.9), TSH 6.81μU/mL (0.3–4.5), and total T3 2.28ng/mL (0.8–2). Thyroglobulin and peroxidase antibodies were positive (455IU/mL (0–115) and 274IU/mL (0–32) respectively). The results of alpha subunits of glycoprotein hormones, cortisol, estradiol, gonadotropins, and prolactin were normal. Measurements of T3 and T4 antibodies provided normal results. Ultrasound images were consistent with multinodular goiter. The biggest nodules were 1cm and 1.3cm in size and had a benign appearance in ultrasonography. Based on these data, resistance to thyroid hormone was suspected. The patient was asked to provide laboratory tests from first-degree relatives. Tests from her brother, who was also symptom-free, also showed high free T4 and TSH levels (3.98ng/dL and 6.72μU/mL respectively). Finally, a genetic study was performed by sequencing exons 3–10 of the THRB (thyroid hormone receptor beta) gene, and a heterozygous mutation was found in exon 10, consisting of c.1357C>T;p.Pro453Ser.1
In subsequent visits, TSH levels ranged from 6.6 to 8.1, and free T4 levels from 2.8 to 3.2. Low TSH levels were found three years after diagnosis, and repeated tests showed TSH <0.014, free T4 >7.77, and TSI 21.12IU/L (positive >1.5). The patient reported a moderate weight decrease (2kg in four months), a slight increase in nervousness, palpitations, and occasional tremor. No ophthalmopathy was found. A thyroid scan (Tc99) showed a thyroid gland of normal location and shape with a diffusely increased uptake.
GBD was diagnosed, and treatment was started with methimazole 30mg daily in descending doses. High transaminase levels were found three months later (see Table 1), and treatment was therefore discontinued after showing improvement. Propylthiouracil was instead administered, but markedly high transaminase levels again occurred, associated with normal bilirubin levels, normal liver ultrasound examination, negative serologic tests for hepatitis B and C, and negative LKM, ANA, AMA antibodies. Propylthiouracil was discontinued based on the diagnosis of thionamide-induced toxic hepatitis, and the patient was referred for radioiodine treatment.
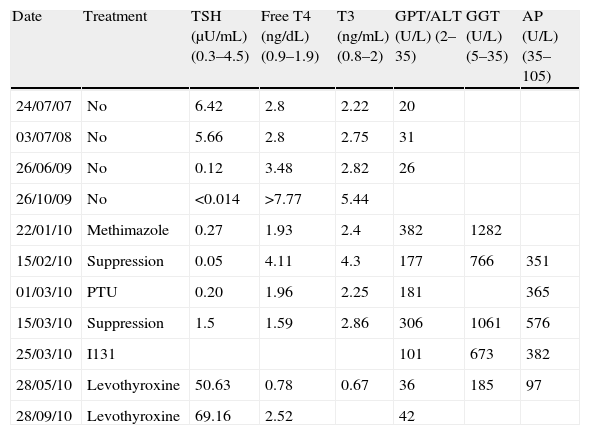
Changes over time in laboratory results.
| Date | Treatment | TSH (μU/mL) (0.3–4.5) | Free T4 (ng/dL) (0.9–1.9) | T3 (ng/mL) (0.8–2) | GPT/ALT (U/L) (2–35) | GGT (U/L) (5–35) | AP (U/L) (35–105) |
| 24/07/07 | No | 6.42 | 2.8 | 2.22 | 20 | ||
| 03/07/08 | No | 5.66 | 2.8 | 2.75 | 31 | ||
| 26/06/09 | No | 0.12 | 3.48 | 2.82 | 26 | ||
| 26/10/09 | No | <0.014 | >7.77 | 5.44 | |||
| 22/01/10 | Methimazole | 0.27 | 1.93 | 2.4 | 382 | 1282 | |
| 15/02/10 | Suppression | 0.05 | 4.11 | 4.3 | 177 | 766 | 351 |
| 01/03/10 | PTU | 0.20 | 1.96 | 2.25 | 181 | 365 | |
| 15/03/10 | Suppression | 1.5 | 1.59 | 2.86 | 306 | 1061 | 576 |
| 25/03/10 | I131 | 101 | 673 | 382 | |||
| 28/05/10 | Levothyroxine | 50.63 | 0.78 | 0.67 | 36 | 185 | 97 |
| 28/09/10 | Levothyroxine | 69.16 | 2.52 | 42 |
AP, alkaline phosphatase; PTU, propylthiouracil.
She was administered 12mCi of radioiodine six months after the start of antithyroid medication. One month later, hormone levels consistent with hypothyroidism (TSH 50.63 and free T4 0.78) were found, and replacement therapy was started with levothyroxine. The patient still had elevated TSH levels of 69.16at four months but a high free T4 value of 2.05 (0.9–1.9), and levothyroxine dosage therefore continues to be adjusted (her current dose is 175μg daily). The patient is symptom-free.
RTH occurs in one out of every 40,000 live newborns and is virtually always inherited as a dominant autosomal trait. In 85% of cases it is caused by mutations in the THRB gene. Most patients are clinically euthyroid, but may have goiter, hyperactivity, tachycardia, delayed growth, or learning difficulties.2 Both our patient and her brother were symptom-free, and the condition was incidentally found.
A TSH-secreting pituitary adenoma should be considered in differential diagnosis. A TRH stimulus test was not performed in our patient because family history and normal subunit alpha ruled out an adenoma, and the condition was finally confirmed by study of the THRB gene.
The most common form of thyroid autoimmunity is Hashimoto thyroiditis, affecting approximately 3% of the population. Several articles showing the coexistence of Hashimoto thyroiditis with RTH have been published in recent years.3–5 This has led to various hypotheses being postulated about the relationship between thyroid autoimmunity and RTH. Thus, it has been suggested that chronic stimulation by TSH in these patients could induce an immune response, increasing the coexistence of both conditions.6 A review of 130 families with 330 individuals affected by RTH showed an increased probability of developing autoimmune thyroid disease in individuals with RTH having a mutation in the THRB gene as compared to those with no mutation. This appears to show that the association between RTH and autoimmune thyroid disease is not a mere coincidence.7
Unlike in Hashimoto thyroiditis, we have only found references to two patients with RTH who developed GBD. One of these was treated with thionamides, which achieved remission of the condition.8 The other patient was treated with radioiodine and subsequently experienced hypothyroidism requiring high levothyroxine doses.9
Because of diagnostic difficulties, patients with RTH and Graves-Basedow disease are likely to be underdiagnosed or to be detected only when they experience hyperthyroidism, which makes exact subsequent diagnosis difficult. On the other hand, patients with RTH have not uncommonly been erroneously treated by thyroidectomy or radioiodine as if they had hyperthyroidism, which may especially occur in the presence of thyroid autoantibodies.
In should be noted that our patient also had an uncommon condition, methimazole-induced toxic hepatitis. Adverse reactions to antithyroid drugs occur in less than 10% of cases and are usually minor (skin rash, joint pain, etc.).
Major side effects, including agranulocytosis (0.2%-0.5%), vasculitis, lupus-like syndrome, and hepatotoxicity, are very rare.10 Cholestatic conditions (dissociated cholestasis in the reported case), resolved upon drug discontinuation, usually occur.11 Our patient rapidly improved after methimazole was discontinued, but experienced a cross-reaction when this was replaced by propylthiouracil and finally required treatment with radioiodine. Radioiodine therapy caused hypothyroidism, and the patient required high levothyroxine doses in an attempt to normalize TSH levels. In the presence of hypothyroidism, either induced by Hashimoto thyroiditis or after surgery or radioiodine therapy, requiring high levothyroxine doses to be controlled, RTH should be considered in differential diagnosis.
We report the first case of RTH associated with GBD published in Spain. Because of the potential difficulties involved in diagnosing this association, it should be considered in differential diagnosis in patients who have during RTH monitoring clinical or laboratory data consistent with hyperthyroidism, or in those who experience hypothyroidism with high levothyroxine requirements after treatment for GBD.
Please cite this article as: González Cabrera N, et al. Hipertiroidismo por enfermedad de Graves Basedow en mujer con resistencia a las hormonas tiroideas. Endocrinol Nutr. 2012;59:606–11.