There have been in the past decade a growing number of studies relating head trauma to hypopituitarism. This condition may affect the rehabilitation process, and identification of such patients is therefore required. However, the widely different methods used so far for this purpose have provided inconsistent results. The incidence rate of hypopituitarism has probably been overestimated. This review focuses on the impact of head trauma on pituitary function, the diagnostic method, risk factors, and treatment options.
En la última década ha habido una auténtica eclosión de trabajos que relacionan el traumatismo craneoencefálico (TCE) con hipopituitarismo. Esta condición puede afectar el proceso de rehabilitación por lo que se requiere identificar a estos pacientes, pero hasta ahora, la metodología utilizada ha sido muy diversa y los resultados muy dispares. Es probable que la indidencia del hipopituitarismo haya sido sobrevalorada. Esta revisión se centra en el impacto del TCE sobre la función hipofisaria, el método diagnóstico, factores de riesgo y opciones de tratamiento.
Head trauma (HT) represents a highly significant health problem worldwide, with an incidence of 180–250 cases per 100,000 people every year. In developed countries it is the leading cause of death in young adults, and those surviving HT have a high incidence of cognitive, physical, and emotional changes which are permanent in most cases.1,2
The first description relating HT to impaired pituitary function was given by Simmonds almost a century ago.3 However, the occurrence of hypopituitarism after HT was considered to be extremely uncommon, to the point that a series of 595 cases of hypopituitarism published in 1942 reported a history of HT in only four cases.4 However, in the following decades there was a constant description of isolated cases, some of which were compiled in a paper by Edwards and Clark.5 Necropsy studies also detected pituitary changes in up to 30% of patients dying from HT.6,7 All this led to the suspicion that post-traumatic hypopituitarism was not as exceptional as previously thought. The 2000 review by Benvenga et al.7 included 367 cases and represented the definitive inclusion of HT among the etiopathogenetic mechanisms of hypopituitarism.
Since then, an increasing number of studies have addressed the incidence of hypopituitarism after HT, and in 2009 the Spanish Society of Endocrinology and Nutrition issued a consensus document on the subject.8 However, more recent studies appear to suggest that pituitary involvement after HT may have been overestimated.9 Although there are other types of brain insult causing hypopituitarism, whose most common cause is subarachnoidal hemorrhage,10 this review will only focus on HT.
Etiopathogenesis of hypothalamic–pituitary injury after head traumaThe pituitary gland is located inside a bone structure, the sella turcica, which surrounds it completely except for its upper part, where the sellar diaphragm is located. The sellar diaphragm consists of a thick layer of connective tissue through which the pituitary stalk, providing 80–90% of blood flow, passes. This is a portal system that transports the hypothalamic hormones responsible for the regulation of adenohypophyseal secretion. These long pituitary vessels and stalk capillaries are particularly vulnerable to intrasellar swelling caused by traumatic injuries, and the process may therefore lead to pituitary infarction.5,7
The mechanisms proposed to account for the edema and hemorrhage causing pituitary injury include: (1) diffuse brain injury from the collision of the brain structure with the cranial structure, (2) a shock and counter-shock mechanism, which explains brain injury by collision with bone structures located on the side opposite the traumatic collision, (3) bleeding or hematoma secondary to vessel rupture, and (4) penetrating wounds that cause head structures such as skin, hair and bone to act as aggressive factors for cerebral structures.8 Such injuries have been found in autopsy studies of patients dying from HT. Different grades of edema, hemorrhage, and necrosis are seen in such studies, and the three types of injury often coexist.10,12 These represent the primary mechanism of hypothalamic–pituitary injury and may be studied using different imaging techniques.11–14
There are a number of secondary mechanisms derived from the primary lesion. These include high blood pressure, hyperglycemia, intracranial hypertension, hyperthermia, hyponatremia, and hypoxia, which may cause neuronal death through different processes such as increased free radicals, oxidative stress, neuroglycopenia, and increased levels of excitatory amino acids such as glutamate, lactate, and adenosine, which contribute to the maintenance of the primary lesion8.
Interestingly, Tanriverdi et al. have implicated an autoimmune mechanism that could promote the occurrence of hypopituitarism after HT.15 These authors detected the presence of antipituitary antibodies in 46% of subjects who had sustained HT three years before. They also showed the presence of antibodies to be a predictor for the development of hypopituitarism. The authors suggested that autoimmunity may be a factor contributing to the development of hypopituitarism after HT, as it would allow antigens sequestered in the hypothalamic–pituitary tissue to pass into the bloodstream and so trigger a humoral response able to perpetuate neuroendocrine dysfunction. As this is a late response, it would also explain why some patients develop pituitary insufficiency months after HT. The authors speculate that the neuroinflammation mechanism may be involved in late pituitary dysfunction occurring after HT.
Clinical signs and symptomsThe symptoms derived from brain injury mainly consist of neurological and cognitive deficits depending on the severity of the injury. In the acute stage they may partially or totally mask manifestations derived from pituitary insufficiency. The most common are related to changes in arginine vasopressin, antidiuretic hormone (ADH), either diabetes insipidus or syndrome of inappropriate arginine vasopressin secretion (SIADH), and to different salt-losing syndromes. If these conditions are not diagnosed, the life of the patient, already compromised by neurological lesion, may be put at risk. The production of all other pituitary hormones may also be impaired. Clinically, cortisol and thyroid hormone deficiencies may increase the severity of the condition, although they are occasionally part of the adaptation mechanisms to the new clinical situation, which are reversible if the acute stage is overcome.
Subjects who overcome the acute stage often continue to have neurological deficits. In this case, the lack of detection of hypopituitarism may influence recovery, because a lack of thyroxine, growth hormone (GH), and sex hormones may make the rehabilitation process difficult. With regard to GH, it is widely recognized that its deficiency may affect quality of life, bone mineral density, and body composition.7,8 The psychological changes and decreased muscle strength and exercise capacity seen in patients with HT are similar to those found in all other adult patients with GH deficiency. Since GH deficiency after HT may be transient, it is necessary to wait more than 12 months in order to be sure that GH deficiency is permanent and only then can GH replacement therapy in patients with HT be considered.
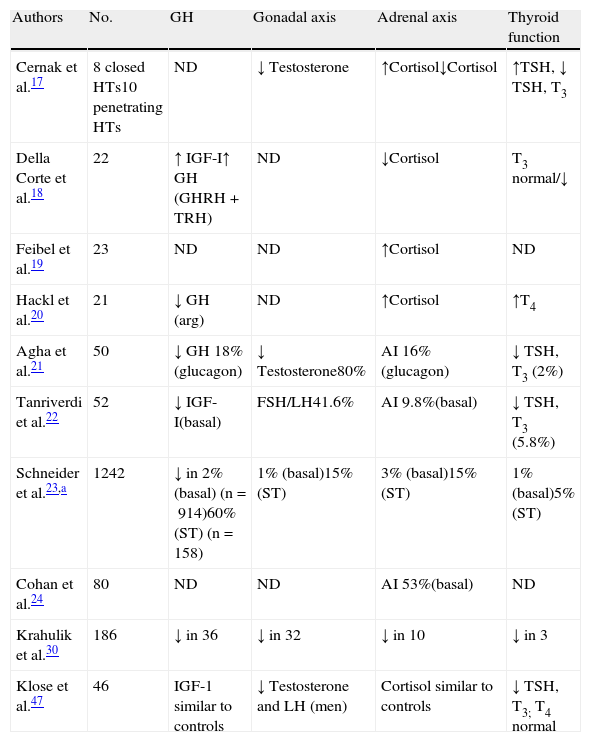
Impact of head trauma on pituitary functionAcute stageThe days immediately following HT are characterized by a number of changes that some authors have encompassed under the term “neuroendocrine dysfunction in the acute stage of HT”.16 The initial data were taken from basal measurements of pituitary hormones and those corresponding to peripheral glands. These studies were not specifically designed to investigate the frequency of hypopituitarism, but were intended to relate the results obtained to trauma severity or other factors susceptible of becoming predictors.17–20 The most recent reports are cohort studies that place more emphasis on hormone deficiencies.21,22 Overall results show a lack of agreement about the nature of neuroendocrine changes occurring after HT, and such discrepancies may be due to differences in patient selection criteria, study design, trauma severity, the timing of hormone measurements, and the methods used.16Table 1 details the changes detected in the different series.
Summary of results obtained in the acute stage of HT.
| Authors | No. | GH | Gonadal axis | Adrenal axis | Thyroid function |
| Cernak et al.17 | 8 closed HTs10 penetrating HTs | ND | ↓ Testosterone | ↑Cortisol↓Cortisol | ↑TSH, ↓ TSH, T3 |
| Della Corte et al.18 | 22 | ↑ IGF-I↑ GH (GHRH+TRH) | ND | ↓Cortisol | T3 normal/↓ |
| Feibel et al.19 | 23 | ND | ND | ↑Cortisol | ND |
| Hackl et al.20 | 21 | ↓ GH (arg) | ND | ↑Cortisol | ↑T4 |
| Agha et al.21 | 50 | ↓ GH 18%(glucagon) | ↓ Testosterone80% | AI 16%(glucagon) | ↓ TSH, T3 (2%) |
| Tanriverdi et al.22 | 52 | ↓ IGF-I(basal) | FSH/LH41.6% | AI 9.8%(basal) | ↓ TSH, T3 (5.8%) |
| Schneider et al.23,a | 1242 | ↓ in 2% (basal) (n=914)60% (ST) (n=158) | 1% (basal)15% (ST) | 3% (basal)15% (ST) | 1% (basal)5% (ST) |
| Cohan et al.24 | 80 | ND | ND | AI 53%(basal) | ND |
| Krahulik et al.30 | 186 | ↓ in 36 | ↓ in 32 | ↓ in 10 | ↓ in 3 |
| Klose et al.47 | 46 | IGF-1 similar to controls | ↓ Testosterone and LH (men) | Cortisol similar to controls | ↓ TSH, T3; T4 normal |
Special mention should be made of adrenal axis involvement, as it may be life-threatening. Cortisol levels increase immediately after HT and gradually decrease to normal after a few days.17,18,20 This activation of the adrenal axis has been related to increased intracranial pressure.19 Adrenal insufficiency, derived from the absence of such activation, occurs in approximately 10–20% of cases,16,22,23 although a study reported a 53% incidence if only basal cortisol is considered.24 Decreased plasma cortisol levels have been related to trauma severity as assessed by the Glasgow Coma Scale (GCS)25 in mild to moderate cases, and the normalization of cortisol was found to be a good indicator of a better prognosis.26,27 By contrast, in severe HTs cortisol has been seen to decrease in the first few days, but subsequently to rise.28 Some patients with severe HT experienced adrenal crises that subsequently showed dramatic responses to glucocorticoid treatment.16 In agreement with this, Cohal et al. reported that high blood pressure requiring treatment was more common in patients with adrenal insufficiency.24
Similarly to cortisol, several studies report activation of the somatotropic axis after trauma. GH increases after HT, and a paradoxical increase has been seen following stimulation with glucose, which normalizes in the recovery phase.28,29 This GH increase was not found in 18% of cases by Behan et al.16 and in 20% in the Tanriverdi et al. study, where it was related to low IGF-1 concentrations.22 Higher percentages were reported by Schneider et al.23 and Krahulik et al.30 Exaggerated GH responses to GHRH and a paradoxical response to TRH related to a poorer prognosis have been reported.18 Hackl et al. reported a lack of GH response to the arginine test, and in very serious cases this was associated with a mortality rate close to 100%.20
As in other acute conditions, suppression of the gonadal axis is very common in HT,17,20,31–33 with prevalence rates higher than 50% in most studies, although the vast majority recover function.
Decreased gonadotropins may reflect an adaptive response to the lesion or characteristic situation of the critical patient but the significance of this is as yet uncertain. The direction of these changes may reflect the existence of an adequate defensive mechanism that decreases the use of energy and substrates corresponding to less vital functions. However, testosterone levels were related to HT severity in some studies,17,31 in contrast to the results of Lee et al.34 Hyperprolactinemia may also be found, although the results are highly variable.35 However, some authors have found a positive correlation with lesion severity.36
Results reported about thyroid axis changes are disparate. Some authors found no changes in thyroxine levels,17,18 while others reported low31 or high19 T4 levels. T3 is usually low or in the lower limit of normal.17 Results discordant with TSH, either low or normal, have also been reported, but values increase after a few days. The significance of these findings is unknown. Decreased levels of thyroid hormones and TSH have been shown to be related to a poor prognosis,17 and a flat response to TRH is followed by a high mortality.20 However, no correlation has been seen in other cases.18
As previously noted, the prevalence of neurohypophysis involvement is variable, although few series have assessed ADH secretion status, either as diabetes insipidus or SIADH. In the Behan et al. study,16 ADH secretion was impaired in 40% of patients. Diabetes insipidus may occur in up to 28% of patients,37,38 associated with more severe cases and with significant brain edema. SIADH also occurs with a highly variable frequency, and has been reported in up to 36% of patients.16 The disparity of these results again reflects differences in patient selection, diagnostic criteria for SIADH, and the time of measurement of sodium levels after HT. SIADH may be detected 48h after trauma, but detection may be delayed for up to three weeks. No correlation exists with either the severity of the trauma or with adenohypophysis involvement. Changes in neurohypophysis are transient in most cases.
The involvement of at least one of the hypothalamic–pituitary axes is common. In the most recent series it ranged from 30% to 60%, and multiple deficits were found in 20–30% of patients.
Our group was able to prospectively assess 46 patients with moderate to severe HT. The preliminary results of 36 patients (26 males and 10 females) in the acute stage were presented at the 2010 SEEN congress.39 All the males had gonadotropin deficiency, 17 (47%) patients had cortisol deficiency, 16 (44%) decreased TSH levels, 22 (61%) hyperprolactinemia, 7 (19%) decreased IGF-1, and 4 (11%) diabetes insipidus. More than one axis was involved in 31 patients. More axes were affected in severe HTs (GCS less than 8) and in patients with a higher grade of diffuse brain lesion.
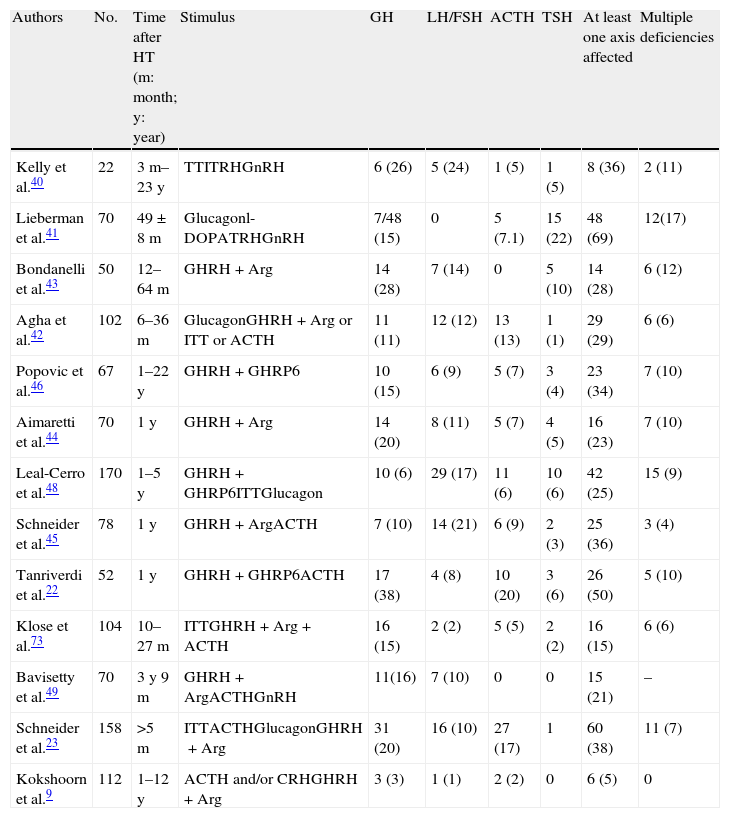
Chronic stageMost series assess hypophyseal function results between 3 and 12 months after HT. Most of these results have been reported in the last decade. Table 2 shows the results of prevalence found in all studies. Early studies used very different methods. The Kelly et al. study40 assessed 22 patients with moderate to severe HT a median of 26 months after injury. The clinical relevance of hormone deficiencies was not reported, and interpretation was complicated by the fact that all patients with hypopituitarism had experienced episodes of hypoxia or hypotension during the acute stage. Lieberman et al.41 recruited their 70 patients from a rehabilitation center, but hypophyseal stimulation tests were only performed in patients with impaired basal hormone levels. GH reserve was tested in 48 patients by glucagon stimulation. A decreased response was found in 7 patients (14.6%), but the result was only confirmed in 5 of them with the l-dopa test. Basal cortisol levels were less than 7mcg/dL in 45% of patients, but only 5 patients did not respond to ACTH stimulation. Impairment in at least one axis was found in 51.4%, and 17.1% had multiple deficiencies. In a prospective study assessing 102 consecutive, unselected patients surviving the acute stage 17 months after trauma, Agha et al.42 used a more consistent methodology. Two stimulation tests were performed in each patient, who was considered to have GH and ACTH deficiency if both tests were abnormal. Twenty-nine percent of these patients had at least one affected axis. GH, ACTH, gonadotropin, and TSH deficiencies were found in 11%, 13%, 12% and 1% of patients respectively, while 13% had hyperprolactinemia. Panhypopituitarism was detected in a single patient. Subsequent studies confirmed these results.22,43–45 It should be noted that although a variable proportion of patients recovered pituitary function at the end of the study, there were also a substantial number of patients in whom pituitary insufficiency occurred at a later time. Similar results were reported in studies by Popovic et al.46 and Klose et al.47 Popovic et al. found hypopituitarism in 34% of patients and multiple deficits in 10%. GH was most commonly impaired (15%), followed by gonadotropins (9%). The authors reported a correlation between impaired GH response and the occurrence of neurocognitive disability and the existence of a depressive condition. The second study found hypopituitarism in 16% of patients, and the authors noted in their conclusions that this prevalence was lower than previously reported and that there could have been confounding factors such as obesity.
Prevalence data of pituitary changes in the chronic stage of head trauma.
| Authors | No. | Time after HT (m: month; y: year) | Stimulus | GH | LH/FSH | ACTH | TSH | At least one axis affected | Multiple deficiencies |
| Kelly et al.40 | 22 | 3m–23y | TTITRHGnRH | 6 (26) | 5 (24) | 1 (5) | 1 (5) | 8 (36) | 2 (11) |
| Lieberman et al.41 | 70 | 49±8m | Glucagonl-DOPATRHGnRH | 7/48 (15) | 0 | 5 (7.1) | 15 (22) | 48 (69) | 12(17) |
| Bondanelli et al.43 | 50 | 12–64m | GHRH+Arg | 14 (28) | 7 (14) | 0 | 5 (10) | 14 (28) | 6 (12) |
| Agha et al.42 | 102 | 6–36m | GlucagonGHRH+Arg or ITT or ACTH | 11 (11) | 12 (12) | 13 (13) | 1 (1) | 29 (29) | 6 (6) |
| Popovic et al.46 | 67 | 1–22y | GHRH+GHRP6 | 10 (15) | 6 (9) | 5 (7) | 3 (4) | 23 (34) | 7 (10) |
| Aimaretti et al.44 | 70 | 1y | GHRH+Arg | 14 (20) | 8 (11) | 5 (7) | 4 (5) | 16 (23) | 7 (10) |
| Leal-Cerro et al.48 | 170 | 1–5y | GHRH+GHRP6ITTGlucagon | 10 (6) | 29 (17) | 11 (6) | 10 (6) | 42 (25) | 15 (9) |
| Schneider et al.45 | 78 | 1y | GHRH+ArgACTH | 7 (10) | 14 (21) | 6 (9) | 2 (3) | 25 (36) | 3 (4) |
| Tanriverdi et al.22 | 52 | 1y | GHRH+GHRP6ACTH | 17 (38) | 4 (8) | 10 (20) | 3 (6) | 26 (50) | 5 (10) |
| Klose et al.73 | 104 | 10–27m | ITTGHRH+Arg+ACTH | 16 (15) | 2 (2) | 5 (5) | 2 (2) | 16 (15) | 6 (6) |
| Bavisetty et al.49 | 70 | 3y 9m | GHRH+ArgACTHGnRH | 11(16) | 7 (10) | 0 | 0 | 15 (21) | – |
| Schneider et al.23 | 158 | >5m | ITTACTHGlucagonGHRH+Arg | 31 (20) | 16 (10) | 27 (17) | 1 | 60 (38) | 11 (7) |
| Kokshoorn et al.9 | 112 | 1–12y | ACTH and/or CRHGHRH+Arg | 3 (3) | 1 (1) | 2 (2) | 0 | 6 (5) | 0 |
Results in brackets are percentages. Arg: arginine; ITT: insulin tolerance test.
Special mention should be made of the Leal-Cerro et al. study48 because of its sample size (170 patients). This was a study of HT over at least a 12-month period placing special emphasis on GH deficiency. The involvement of at least one axis was found in one fourth of patients, and combined involvement in 15 patients (8.8%). Gonadotropins were most commonly impaired (29 patients, 17%), followed by ACTH (11 patients, 6.4%) and TSH and GH (10 cases both, 5.8%). Diabetes insipidus was found in 3 patients only (1.7%). A remarkable characteristic of this series was that there was no prior clinical suspicion of hypopituitarism. The authors stated that there was probably a subclinical deficiency and that its prevalence was also lower as compared to prior series, a fact attributed by them to the longer time interval and to the use of much stricter criteria, particularly for GH.
More recently, four studies of particular interest have been reported. The Bavisetty et al. study49 analyzed 70 cases three and nine months after HT. The authors found that twenty-one percent of cases presented some type of hormonal deficit requiring replacement therapy. This was related to lesion severity. GH deficiency, found in 16% of patients, was associated with greater impairment of quality of life, depressive status, and disability. In addition, minor abnormalities defined as subclinical were found in an additional 29% of patients, and the authors concluded that their clinical significance should be assessed in subsequent studies. In the Krahulik et al. study,30 results at 3, 6, and 12 months were analyzed, in addition to the abovementioned acute stage data. Fifty-five percent of patients with acute stage changes recovered from their hormone deficiencies, 8% recovered at six months, and two patients at 12 months. By contrast, an impairment not present in the acute stage was diagnosed in 11 patients only. The somatotropic and gonadotropic axes were most commonly affected.
The results of the recently reported study by Schneider et al.,23 providing data from 825 HT seen at 14 German centers and one Austrian hospital, are of particular interest. In this study, the prevalence of any deficit was 38% if the results of basal studies (free T4, cortisol, and IGF-1 in all patients and testosterone in males) only were considered, or 32% when the diagnosis was made by the medical team in charge of the patient. However, when stimulation tests were performed (in only 158 patients) to test GH and ACTH, prevalence increased to 60%. Patients were studied at least five months after the trauma.
Finally, as a proof of the widely disparate results reported, Kokshoorn et al.9 studied 112 patients, of whom 30% had GCS lower than 7, and in whom only one gonadotropin deficiency, two adrenal insufficiencies, and three GH deficiencies were detected. These authors used insulin-induced hypoglycemia to assess GH and ACTH reserve or, if contraindicated, ACTH/CRH or GHRH. These stimulation tests were performed in 80% of patients. Cut-off points were adjusted for body mass index (BMI).50 The authors explained the disagreement of their results with those from prior series by the lack of adjustment for BMI in the latter. In fact, according to their results, the six patients with pituitary involvement had BMI values significantly higher than the rest. Differences in prevalence may be explained by the performance of the assessment at different times after HT, and the authors recommend that tests be done at least 12 months after trauma.
Post-traumatic hypopituitarism in childhood and adolescenceThe incidence of HT between five and 14 years of age is high. Seventy-five percent of injuries are unintentional and mainly include traffic accidents, falls, and sports injuries, most commonly in adolescence.16 However, minor trauma and some cases of abuse, which may not be considered at the time of taking the clinical history, should be taken into account as significant causes of HT in children under one year of age. In the first systematic study in children, conducted by Einaudi et al. in 2006,51 22 and 30 children respectively were retrospectively and prospectively studied. Sixty-six percent of them were re-evaluated one year later. Impaired hormone responses were found in 16% of retrospective cases and in only 8% in the prospective study. GH deficiency was most common. The prevalence of hypopituitarism was lower than that reported for adult populations. By contrast, in another study of 26 children with an average course of 30 months, Niederland et al.52 reported a 61% prevalence of pituitary involvement. GH deficiency was found in 42% of children. However, no differences in height were found between children with and without GH deficiency. The cut-off point (less than 7ng/mL) was probably too high for l-dopa and, thus, the prevalence of GH deficiency may be greatly overestimated.
A review by Acerini et al.53 reported 20 patients selected from a literature review, of whom 85% had GH deficiency, 75% TSH deficiency, 55% ACTH deficiency, 80% gonadotropin deficiency, and 10% diabetes insipidus. The percentages reported in this series are difficult to extrapolate, and this review quotes unpublished data from a KIGS compilation where only 141 out of more than 23,000 patients with GH deficiency had a history of HT. The authors emphasized the need for prospective studies to assess this apparently low prevalence. Subsequent studies provided results that confirmed this lower prevalence as compared to the adult series. In a prospective study of 117 severe HTs (GCS less than 8),54 four patients had previously been diagnosed at the time of assessment. Another 29 patients were studied based on the results of a questionnaire. Nine of these had pituitary involvement, which was multiple in four of them. In their conclusions, and despite this moderate prevalence, the authors recommended the assessment of pituitary function in patients with severe trauma. Two studies with very different results have more recently been published. Khadr et al.55 conducted a retrospective study of 33 patients and found no significant deficiencies, although suboptimal GH and cortisol responses were detected in seven and 11 patients respectively. Replacement therapy was not required. Finally, Moon et al.56 assessed 20 patients five years after HT in a case–control study. They found gonadotropin deficiency in a single patient, who refused to continue in the study. The authors recommended that pituitary function tests should not be routinely performed in children with HT.
Assessment of pituitary function after head traumaHT may be rated as mild, moderate, or severe. Most studies included patients from the last two groups (GCS ranging from 3 and 13 or radiographic evidence of brain injury). Patients with severe or moderate trauma are more likely to continue to have greater morbidity than milder cases and may benefit from routine assessment to rule out and, if appropriate, treat hypopituitarism. Risk factors such as diffuse axonal damage, skull base fractures, and older age are a priority when deciding whether to perform a pituitary test. Milder cases should only be studied if clinical evidence exists.
In the acute stage, adrenal insufficiency should not be overlooked, because it may be life-threatening. According to the criterion established by Agha et al.,35 basal cortisol levels less than 200nmol/L suggest ACTH deficiency and should be treated with adequate glucocorticoid doses. If cortisol levels range from 200 to 400nmol/L, a clinical assessment should be made, and treatment given if hypotension, hyponatremia, or hypoglycemia occur. According to Kokshoorn et al.,57 however, these cut-off points should be changed to 100 and 500nmol/L respectively. Dynamic tests are not helpful in the acute stage in these patients, and basal cortisol measurement is recommended in the first 7 days.16 The SEEN consensus document8 also emphasizes the necessity of assessing the adrenal axis and the importance of ruling out water and electrolyte (diabetes insipidus, SIADH, and other conditions characterized by salt loss) and thyroid axis changes. However, thyroid function assessment is not a priority for other groups.16,35 There is virtual unanimity that, in the acute stage, GH and gonadotropin secretion tests should not be done beyond basal levels of pituitary hormones and their corresponding peripheral hormones.
In the chronic stage, dynamic tests are needed to assess the somatotropic and adrenal axes. Several groups agree that all moderate or severe HTs (GCS 3–13) should be assessed, while the assessment of mild HTs should only be required if there are signs and symptoms suggesting pituitary involvement.8,10,16,35,44,46–48
The SEEN consensus8 recommends that GH secretion be measured from 12 months, or earlier if clinical evidence or suspicion or other documented deficiencies exist. A test assessing pituitary GH reserve is required for this purpose because the isolated measurement of GH or IGF-1 has no diagnostic value. In patients with clinical signs consistent with hypopituitarism and a history of TH, both basal hormone and GH reserve tests are mandatory. The 2007 Consensus guidelines for the diagnosis and treatment of adults with GH deficiency58 stated that the insulin-induced hypoglycemia test, the GHRH plus arginine or GHRP6 test, and the glucagon stimulation test are the validated tests in adults for the stimulation of GH secretion. Low IGF-1 levels suggest GH deficiency in the presence of hypopituitarism, but normal IGF-1 levels do not rule out GH deficiency. The Consensus stated that HT is an indication for the use of these tests.
Controversy exists concerning the suitability of these tests for stimulating GH secretion. A study by Schneider et al.59 compared the results of insulin-induced hypoglycemia and GHRH plus arginine tests in 21 patients with HT. The authors reported a 61% discrepancy between both tests, as 12 patients were rated as GH-deficient using GHRH plus arginine despite having a normal response in the insulin-induced hypoglycemia test. This discrepancy was partly attributed to BMI, which was higher than 28 in all these patients. When the cut-off points were adjusted (4.2, 8.0, and 11.5ng/mL for obese, overweight, and normal weight patients respectively), the difference decreased to 14.3%, with discrepant tests in only three patients.
The combined administration of GHRH and arginine or GHRP6 has been known to be a safe and reliable alternative to the insulin-induced hypoglycemia test for over a decade.60–62 However, although a cut-off point of 9ng/mL has been suggested, the results of Schneider et al. show that BMI should be taken into account as in healthy subjects.63,64 All this may lead to a substantial proportion of obese subjects being rated as GH-deficient using the conventional cut-off point of the GHRH plus arginine test even if they have a normal response in the insulin-induced hypoglycemia test. Thus, if this cut-off point is used in obese subjects, a large number of them will inappropriately receive treatment with GH. Since most patients gain weight after HT, it is important to consider the potential effects of obesity when their pituitary function is assessed.
Unlike other tests, the insulin-induced hypoglycemia test assesses the integrity of hypothalamic and pituitary functions, and has been considered as the reference test for the study of GH and ACTH. However, it cannot be performed in patients with severe cardiovascular disease or uncontrolled seizures, which represents a significant limitation in patients with HT. Some authors have used it with no adverse effects,10,42 while others have used alternative tests. Different cut-off points and test methods have been used, which may be important for defining hormone deficiency. Moreover, some authors perform two tests to confirm abnormalities, while others use a single test. Therefore, the strength of the diagnostic methods varies between the different studies.
The problem is to establish whether post-traumatic hypopituitarism, particularly GH deficiency, has been overestimated in cross-sectional studies. A greater prevalence of post-traumatic hypopituitarism has been documented57 when the glucagon or GHRH-GHRP6 tests are used as compared to the GHRH plus arginine or insulin-induced hypoglycemia tests. In addition, studies using two stimulation tests report lower prevalence rates as compared to studies performing a single test. In addition to the problem of overweight and obesity leading to a diagnosis of GH deficiency in a higher number of patients, another significant aspect is that many patients have a single deficiency, and a single stimulation test may be inadequate for providing reliable results. However, while a second test would be very helpful if the result was confirmed, it could cause confusion if discrepant results were obtained.
Similar problems exist in the assessment of the adrenal axis. Thus, prevalence rates of adrenal insufficiency ranging from 5% to 19% have been found to depend on the test used.57 Basal cortisol levels are helpful if they are lower than 100nmol/L or higher than 500nmol/L. The ACTH test has been shown to be effective in severe cases,65,66 but its results may not be completely reliable in mild adrenal insufficiency.67 The insulin-induced hypoglycemia test continues to be the reference test, and has the advantage of simultaneously assessing GH and ACTH reserves. The results vary depending on the test used.
The diagnosis of central hypothyroidism is based on free T4 levels, which show great individual variation.68 It is therefore difficult to establish a cut-off point, because a given free T4 value may suggest hypothyroidism in one patient but not in another. Basal TSH has a limited value for the diagnosis of central hypothyroidism because normal or even high TSH levels may be detected.69 TSH response to TRH stimulation has limitations because patients with central hypothyroidism may have no TSH response to TRH or show increased TSH levels that overlap with the results seen in healthy subjects. TSH response depends on TRH dose and is higher in women, while it declines with age.70
As regards the assessment of the gonadal axis, the measurement of basal FSH and LH levels may be sufficient in men and postmenopausal women. In premenopausal women, the time of the menstrual cycle should be considered. The choice of the time from HT occurrence for testing gonadal function is also important. Before six months, the results obtained may be transient,71 while those found after 12 months may be considered as definitive in most cases.57
To sum up, because of the high incidence of HT in the general population and the incidence of hypopituitarism in this group of patients, and in agreement with the SEEN Consensus Document, it would be advisable to routinely record in clinical histories any prior HT that warrants a hormone profile including FSH/LH, testosterone, ACTH, plasma cortisol, TSH, free T4, prolactin, and IGF-1. If there are hormonal changes or clinical evidence exists, the assessment of GH and the adrenal axis is mandatory. The insulin-induced hypoglycemia test continues to be the reference test, but will not be feasible due to contraindications in a substantial number of patients. The ACTH stimulation test and combined tests using GHRH plus arginine or GHRP6 may be performed, although they may not be available in many centers. If this occurs, stimulation with glucagon is a perfectly valid alternative, which will also allow for assessing both hormonal axes.
PredictorsIn order to identify those patients with HT who are most likely to experience hypopituitarism, an attempt has been made to find out potential predictors. In the pioneering Benvenga et al. study,7 most patients with pituitary involvement had been in a traffic accident and sustained skull fractures, the condition correlated to coma occurrence and duration, and hemorrhagic lesions were found in the hypothalamic–pituitary area.
The GCS25 is the scale most commonly used to assess the severity of brain injury in clinical practice. The GCS assesses eye opening and verbal and motor response in a scale ranging from 3 to 15. HTs are classified as severe, moderate, or mild depending on whether the GCS score is 3–8, 9–13, or 14–15 respectively. In some studies, no relationship was reported.22,42,44–46,72 In other studies, however, the more common the hypopituitarism, the more severe was the HT.30,43,73 The Schneider et al. review10 also reported a correlation between the occurrence of hypopituitarism in the chronic stage and HT severity according to the GCS. In the German registry,23 no relationship was found between hormone deficiencies and BMI, the GCS, the Glasgow Outcome Score, or the Hunt and Hess scale, but a relationship was found with age, which was lower, the higher the number of axes affected. When the group of cases where only GH and ACTH stimulation tests were performed was analyzed, a relationship was found with severity according to the GCS. The presence of diabetes insipidus in the acute stage did not correlate with adenohypophyseal changes, but correlated with severity of coma and brain edema.37
Some studies found a relationship between radiographic findings and hypopituitarism. Thus, brain edema has been seen to be a potential predictor,40,49,74 while other authors have found an increased occurrence of empty sella syndrome.30
Diffuse axonal lesion has also been related to hypopituitarism in some series.45 The same authors also found an increased incidence of skull base fractures, similar to the Bondanelli et al. series.43 Finally, intracranial pressure elevation has also been reported to be a predictor of hypopituitarism.73
TreatmentReplacement therapy for hypopituitarism in patients who have sustained HT is part of the therapeutic measures for each particular case. Replacement therapy reverses symptoms and normalizes the risk factors associated with hormone deficiencies. In HT, however, brain injury may be subtle, and in many patients only subclinical pituitary changes occur. Moreover, patients often have other multiple sequelae of trauma such as depressive states, neuropsychological changes, or personality changes. Thus, it is by no means clear that they will benefit from replacement therapy to the same extent as patients with more classical causes of hypothyroidism.
Acute stageBecause of the potentially serious consequences of impairment in the adrenal and thyroid axes or neurohypophysis, it is important to adequately treat these patients if clear clinical and biochemical evidence exists. Gonadotropin deficiency is often transient in the acute stage,10 and is therefore no indication for sex steroid replacement.
Treatment with desmopressin is warranted in any patient with diabetes insipidus, i.e. urinary frequency with decreased urinary osmolality and elevated plasma osmolality. There are no clear-cut criteria for glucocorticoid replacement in this stage. Despite the negative results of the CRASH study on 10,000 patients with HT, the only one to suggest that mortality was more than two-fold greater in the group treated with glucocorticoids than in the untreated group,75 it appears reasonable, as stated in the SEEN Consensus,8 to treat patients with marked cortisol deficiency (levels less than 100nmol/L) with high hydrocortisone doses, approximately 50mg every 6h as a minimum. When cortisol levels range from 100 to 200nmol/L, a replacement dose of approximately 25mg daily may be used. If cortisol levels range from 200 to 500nmol/L, most authors recommend treatment if hypotension, hyponatremia, or hypoglycemia exists.
In the acute stage, treatment of central hypothyroidism is warranted if T4 levels are lower than 0.7ng/mL on two occasions separated by more than 48h.
Treatment with GH in this stage is not warranted in any case.
Chronic stageAs in other causes of hypopituitarism, persistent hormone deficiency should be considered to exist when both the peripheral and pituitary hormone concentrations are lower than normal. In the intermediate stage, from 3 to 6 months, only clinically relevant deficiencies should be treated.
GH deficiency deserves special attention. Treatment with GH is indicated for adults with severe GH deficiency and has clearly documented benefits.76 Thus, in adults who have sustained HT and have persistent GH deficiency, both clinical and biochemical, associated with other pituitary deficiencies, treatment with GH should be considered, but always more than 12 months after the trauma.
Preliminary studies with small sample sizes have shown GH to have neurocognitive and psychiatric benefits in GH-deficient patients who have sustained HT.77–79 On the other hand, Tanriverdi et al.80 reported that two retired boxers with GH deficiency experienced substantial improvements in lipid profile, quality of life, and body composition. However, further studies are needed to assess the benefit of treatment with GH in terms of rehabilitation, functional recovery, body composition, and neuropsychiatric function of these patients.
Because of the probability of the recovery of pituitary function in a substantial number of patients, regular re-evaluation in needed in patients with hypopituitarism after HT.
ConclusionsHT should be considered as a significant health problem because of its high incidence in the general population. Patients who have sustained HT will experience, in addition to a substantial mortality, high grades of physical and psychological disability which will require a long and costly rehabilitation process. For almost a century, HT has been related to the occurrence of hypothalamic–pituitary changes forming so-called post-head trauma hypopituitarism. A substantial number of studies reporting highly variable prevalence rates of deficiencies of pituitary hormones have been published in the past decade. Such different figures are mainly due to the use of different methods, which may have caused an overestimation of hypopituitarism after HT. There have been however a substantial number of cases with truly impaired pituitary hormone secretion, which increases the importance of adopting standardized test methods to obtain valid results concerning the impact of HT on pituitary function. This will make it possible to assess in this group of patients the results of hormone replacement therapy and its potential benefits in terms of rehabilitation and functional recovery.
FundingThe author received a grant from Pfizer Inc. to study a group of these patients.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Obiols Alfonso G. Efectos del traumatismo craneoencefálico sobre la función hipofisaria. Endocrinol Nutr. 2012;59(8):505–15.