Overnutrition may lead to obesity. Maternal obesity may affect fertility not only via anovulation, but also through direct effects on oocytes and preimplantation embryos, indicating that the periconceptional period is sensitive to conditions of overnutrition. The periconceptional period includes from folliculogenesis to implantation. Animal model studies suggest that oocytes derived from obese females usually have a small size and mitochondrial abnormalities. These disruptions are probably induced by changes in the components of the ovarian follicular fluid. Experimental evidence also suggests that obesity may affect the microenvironment in oviducts and uterus, resulting in development of preimplantation embryos with reduced cell numbers and upregulation of proinflammatory genes. However, further research is needed for in-depth characterization of the effects of maternal obesity during the periconceptional period.
La sobrenutrición puede ocasionar obesidad. La obesidad materna puede afectar la fertilidad no solo a través de la anovulación, sino también por medio de efectos directos en ovocitos y en embriones en la fase de preimplantación, indicando que el periodo de periconcepción es sensible a condiciones de sobrenutrición. El periodo de periconcepción abarca desde la foliculogénesis hasta el momento de la implantación. Estudios en modelos animales indican que ovocitos derivados de hembras obesas usualmente muestran una talla pequeña y anormalidades mitocondriales. Estas perturbaciones son probablemente inducidas a través de alteraciones en los componentes del fluido folicular ovárico. La evidencia experimental también indica que la obesidad puede afectar el microambiente en oviductos y útero, lo cual conlleva al desarrollo de embriones en la fase de preimplantación con un número reducido de células y con una regulación ascendente de genes proinflamatorios. Sin embargo, se necesita más investigación para una caracterización a fondo de los efectos de la obesidad materna durante el periodo de periconcepción.
Overnutrition occurs when the bioavailability of one or more macronutrients and/or micronutrients exceeds the amounts needed for normal physiological and metabolic activity, usually leading to overweight or obesity.1 Obesity is currently a public health problem worldwide.2–5 The etiology of obesity is multifactorial,6–9 and its pathophysiology has not been fully elucidated yet.10,11 In addition to causing health problems associated with cardiovascular and metabolic diseases,5,12,13 obesity may also cause fertility problems.14 The available data suggest that the chance of spontaneous conception is decreased in obese females, even in the presence of menstrual cycles.15–19 In addition, obese females in assisted reproduction programs usually have an unfavorable response to them. This has been suggested by three recent meta-analyses that examined more than 90 research articles related to the effect of a high body mass index on the results of assisted reproduction techniques.20 In fact, most obese women with fertility problems are advised to lose weight before they undergo assisted reproduction programs.21 In obese women, infertility is usually attributed to lack of ovulation.22,23 However, several research lines have recently shown that both oocyte and embryonic development may also be negatively affected by obesity during the preimplantation period, suggesting that the periconceptional period is sensitive to overnutrition conditions. Generally speaking, the periconceptional period comprises from folliculogenesis to the time of implantation.1 The purpose of this article is to provide an updated review of the effects of maternal overfeeding on the periconceptional period. Most of the information discussed comes from obesity models, the most commonly used in overnutrition studies.
Effect of obesity on ovarian follicular developmentThe infertility seen in obese females is usually associated with anovulation, which is in turn often related to polycystic ovary syndrome.24,25 In a mouse model of diet-induced obesity, lack of ovarian follicular development and ovulation was seen, suggesting the absence of estrous cycles.26 Infertile mice with diet-induced obesity also had increased serum levels of metabolic hormones (i.e. insulin, leptin, and adiponectin) and metabolites (glucose).26 The pathophysiology of anovulation under conditions of obesity is not fully understood, but metabolic changes such as hyperinsulinemia and hyperleptinemia are known to be common characteristics in obese women with or without polycystic ovary syndrome.27,28 Experiments in rats suggest that high leptin levels may prevent ovulation.29,30 However, ovulation may occur under overnutrition conditions, because term pregnancies are common in obese women, including those with morbid obesity.1 Ovarian folliculogenesis is a prerequisite for the ovulation process31 which may be present in obese individuals, although it is deficient in most cases. For example, a low antral follicle count is an ovarian characteristic present in obese women32 and in animals that are fed diets rich in lipids and cholesterol.33 A low number of ovulations or a decreased ovarian follicle count has also been documented in obese genotypes such as obese New Zealand mice,34 obese Zucker rats,35 and Mediterranean Iberian pigs.36 This deficient ovarian follicular activity is quite probably the reason for the low number of oocytes obtained from obese women on in vitro fertility treatments,37 and may be related to the low antimüllerian hormone levels usually detected in obese women.38–40 Other authors believe that serum antimüllerian hormone levels are not related to obesity.41 However, it is known that leptin, which is usually increased in obese individuals, may suppress gene expression of the antimüllerian hormone in granulosa cells through the Janus kinase 2/signal transducer and activator of transcription 3 pathway.42
An additional characteristic in mice with diet-induced obesity is an increased proportion of apoptotic ovarian follicles,43 especially in granulosa and cumulus cells.44 In an overnutrition model of rabbits fed diets rich in lipids and cholesterol, increased ovarian follicular atresia was also found.33 Similarly, obese Zucker rats (i.e. a genetic obesity model) also showed an increased proportion of atretic follicles together with increased insulin levels.35 In this genetic obesity model, follicular atresia was associated with the accumulation of Forkhead box protein O1 in the nucleus of granulosa cells which showed signs of apoptosis.35 Forkhead box protein O1 is a transcription factor that plays a primary role in energy metabolism and cell death, and is largely regulated by insulin.45,46 This is an example where a nutritional mediator may directly affect ovarian cells. However, some nutritional mediators may indirectly affect ovarian follicular development. One such mediator is leptin, due to its relationship with vascular endothelial growth factor, which has a primary role in the regulation of ovarian angiogenesis.47 In fact, protein expression of vascular endothelial growth factor in ovaries may be increased through intraperitoneal leptin injections in mice.48 In humans, leptin concentrations in ovarian follicular fluid may be negatively correlated to intrafollicular oxygen levels49 and to blood flow in ovarian stroma.50 As oxygenation levels in ovarian follicular fluid greatly depend on perifollicular vascular development,51 it is thought that, in the presence of obesity, high leptin levels may interfere with the supply of oxygen and regulatory elements critical for folliculogenesis.25 However, this hypothesis needs to be verified in experimental studies.
Although the information available suggests that ovarian follicular development is impaired in overnutrition, more detailed studies addressing the effect of obesity on ovarian folliculogenesis are needed. It is important to analyze the ovarian microenvironment (e.g. metabolic and proteomic), including intrafollicular fluid and granulosa cells, because these will provide oocyte competency markers that could have clinical application in reproductive medicine.
Effect of obesity on oocyte developmental capacityFrom the periconceptional viewpoint, oocyte developmental capacity refers to the ability of the oocyte to mature during the last stages of folliculogenesis, achieve the fertilization process, and develop up to the blastocyst stage.1 Mice with diet-induced obesity showed a decreased oocyte count with rupture of the germinal vesicle, which suggests a negative effect of obesity on oocyte maturation.43 Small oocytes are also present in obese women52 and mice with diet-induced obesity.43 If oocytes do not reach an adequate size, meiotic maturation is affected, and the polyspermic fertilization rate may be increased.53
The fertilization process is also affected by obesity, because superovulated mice fed a high-fat diet showed a low fertilization rate.44 In a bovine model, the proportion of unfertilized oocytes increased in superovulated obese cows.54 Similarly, fertilization failure rates increased in obese women subject to in vitro fertilization.55 In the latter study, fertilization failure was associated with abnormal meiotic spindles and chromosomal malalignment.55 In fact, mice with diet-induced obesity have an increased aneuploid oocyte rate, combined with an increased rate of morphologically abnormal meiotic spindles and misaligned chromosomes.56
Obesity is partially associated with high lipid consumption, and most animal studies aimed at elucidating the pathophysiology of obesity have used diets with high-fat contents.57 High-fat diets increase lipid content in oocytes, and an excessive increase in lipids is considered a biomarker of lipotoxicity.44 High lipid contents in oocytes are a characteristic usually found in obese animals with reproductive problems.44,58 In fact, in vitro exposure of mouse oocytes to human follicular fluid with high lipid concentrations resulted in a negative effect on oocyte nuclear maturation.59 Experiments in mice suggest that such oocyte lipotoxicity is associated with an impaired expression of stress marker genes in endoplasmic reticulum, such as activating transcription factor 4 (ATF4) and heat shock 70-kDa protein 5 (glucose-regulated protein, 78kDa).44 In the latter study, the expression of both genes increased in cumulus–oocyte complexes, while only ATF4 increased in granulosa cells.44 Granulosa cells from obese women also showed increased ATF4 expression.44
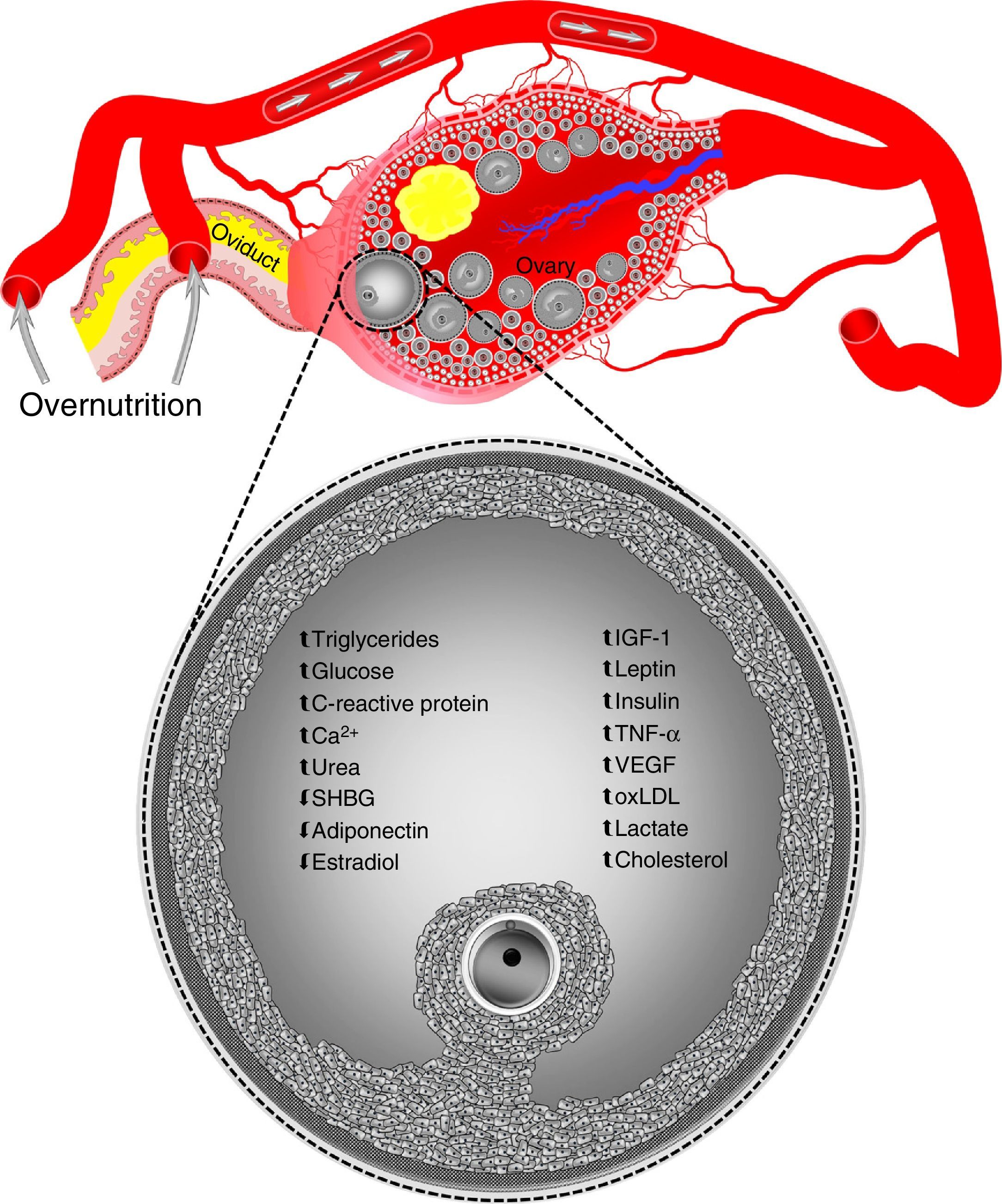
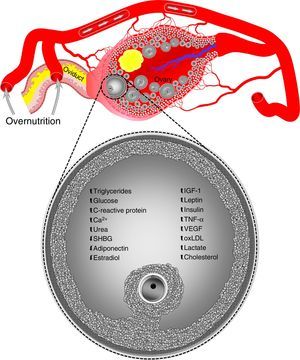
On the other hand, oocytes and zygotes from mice with diet-induced obesity show a greater production of reactive oxygen species and a more oxidized redox state, as well as abnormal mitochondrial localization.60 Other mitochondrial dysfunctions found in oocytes, but not zygotes, from mice with diet induced obesity include an increased number of copies of mitochondrial DNA and the upregulation of nuclear genes involved in mitochondrial DNA replication and transcription.60 It has been suggested that mitochondrial DNA degradation and replication occurring immediately after fertilization in two-cell zygotes and embryos could explain why altered mitochondrial DNA levels are normalized in zygotes from obese mice.60 In addition, because of the degradation of maternal transcripts that precedes the activation of embryonic genome, the normalization of altered nuclear gene levels associated with mitochondrial biogenesis in zygotes from obese animals reflects the global destruction of maternal messenger RNA during the maternal–embryonic transition.60 However, this is not sufficient to counteract the harmful effects of obesity on oocyte developmental capacity. For example, the in vitro development of zygotes from obese mice is known to be affected, resulting in a decreased blastocyst formation.61,62 Similarly, oocytes aspirated by transvaginal ultrasonography from overfed heifers showed a poor development in in vitro fertilization programs associated with hyperinsulinemia in donor animals.63,64 Such elevated insulin levels may even be similar to those reported in women with polycystic ovary syndrome.63In vivo embryo formation is also reduced in superovulated obese cattle and has been related to high blood levels of insulin65 and insulin-like growth factor 1 (IGF-1).54 Changes in oocytes are induced through modifications in the concentrations of the components of ovarian follicular fluid during overnutrition episodes (Fig. 1).1 However, overnutrition not only affects the intrafollicular microenvironment, but also the reproductive tract microenvironment.
Overnutrition may alter concentrations of the components of ovarian follicular fluid. Data taken from humans, sheep, cattle, and pigs. Ca2+, calcium; IGF-1, insulin-like growth factor 1; oxLDL, oxidized low-density lipoprotein; SHBG, sex hormone-binding globulin; TNF-α, tumor necrosis factor-alpha; VEGF, vascular endothelial growth factor. Blood vessels, ovary, ovarian follicles, and oviduct are not drawn to scale.
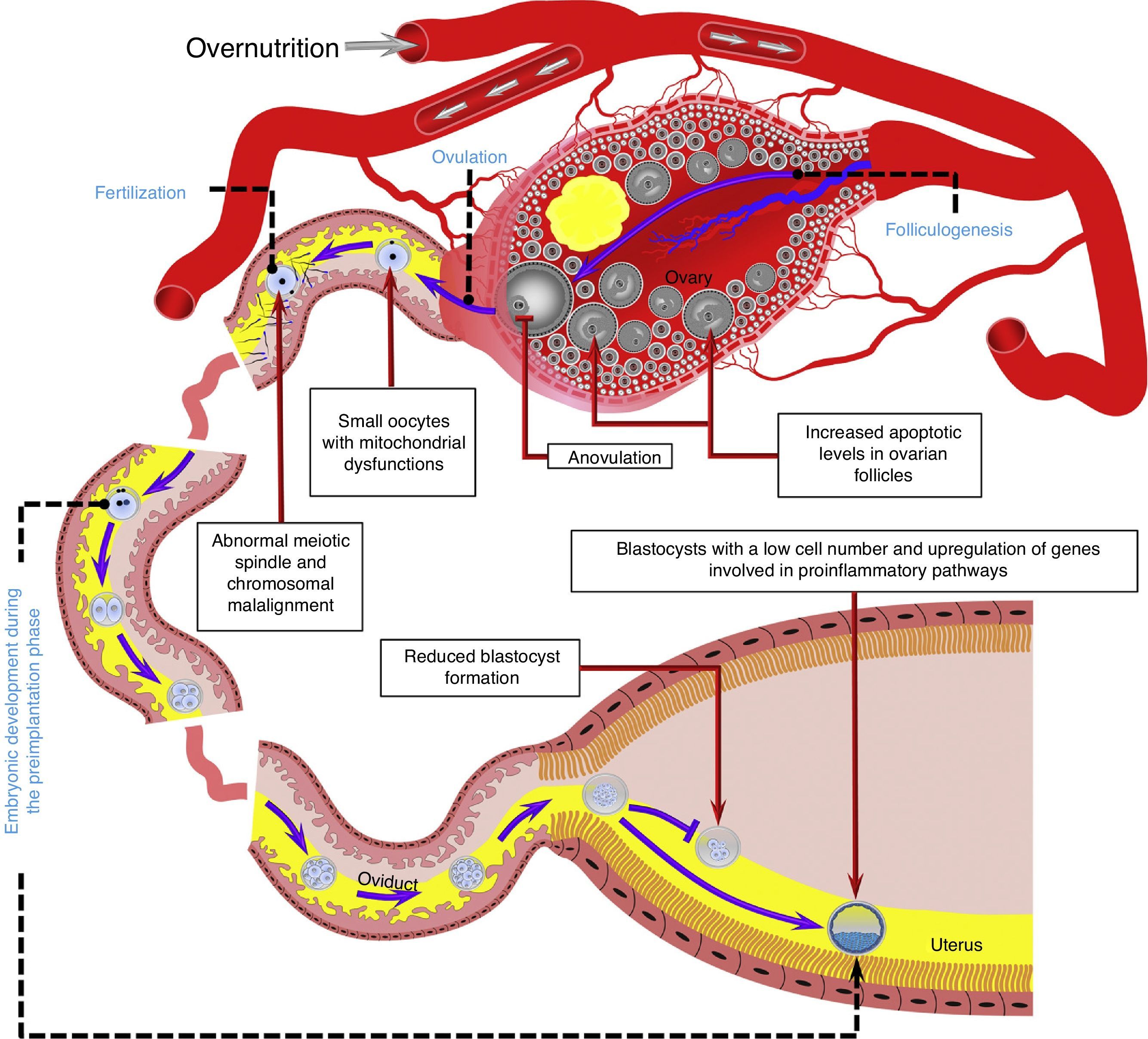
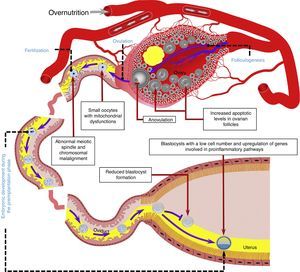
The oviductal microenvironment may be affected by obesity. For example, it is known that leptin concentrations in oviductal luminal fluid are increased in obese mice.60In vitro studies in mice have shown that a high leptin concentration in embryonic culture medium may decrease blastocyst formation66 and induce DNA fragmentation in the resulting embryos67 (Fig. 2).
Overnutrition may negatively affect several reproductive processes during the periconceptional period, including folliculogenesis, ovulation, oocyte quality, and fertilization, as well as embryo development in the preimplantation phase. Model based on data collected from humans,32,52,55 ruminants,54,63,65 and rodents.43,44,56,60,72 The reproductive tract is representative of ruminant and rodent species. Blood vessels, gametes, embryos, and reproductive structures are not drawn to scale.
Colony-stimulating factor 2 (CSF2 [granulocyte–macrophage]) is an important regulator of early embryonic development, and both its gene and protein expression in the oviduct are decreased in obese cows.68 The relevance of these results is not clear, because decreased CSF2 expression was mainly seen in the oviductal ampulla68 and this structure is more relevant for the fertilization process than for the first cell divisions of the embryo.69 No literature reports suggesting an effect of CSF2 on the fertilization process are available.
The uterus may also be affected by overfeeding. The low embryo production reported in obese cows is associated with increased IGF-1 levels in uterine luminal fluid.54In vitro studies suggest that high IGF-1 concentrations may increase apoptotic levels and alter cell allocation in bovine blastocysts.70 Such changes include the formation of blastocysts with excess cell proliferation in the inner cell mass, which may increase the risk of early pregnancy loss.71 However, the latter most probably occurs during short-term overnutrition, in which obesity does not develop and IGF-1 levels are not chronically increased, because under obesity conditions the number of cells in blastocysts does not increase, but rather decreases.54 The low number of cells in bovine embryos is associated with reduced protein expression of the IGF-1 receptor.54 This decrease in protein expression in the IGF-1 receptor has also been reported in blastocysts from mice with diet-induced obesity.43
Experiments in rats have shown that obesity induces an extensive upregulation of genes involved in proinflammatory pathways in the uterus.72 This proinflammatory reaction induced by obesity also occurs in the blastocyst and is associated with lipid accumulation in the uterus and the blastocyst.72 As stated above, hyperlipidemia is considered an indicator of lipotoxicity, and an increase in the lipid content in bovine embryos results in the embryo being given a dark appearance, and embryos with a dark appearance may decrease the pregnancy rate in embryo transfer programs in cattle.73
Controversy exists as to the significance of endometrial receptiveness in obese women, because in oocyte donation models some authors have reported a negative impact on the results of in vitro fertilization,74 while other authors have found no effect.75 Animal models, however, show that obesity is harmful for the uterine environment. It should be taken into account that such studies were retrospective analysis conducted on data routinely collected from women with fertility problems. This type of retrospective analysis has been criticized based on evidence that it may provide implausible results.76 One example is the presumed positive effect potentially exerted by male obesity on implantation rates in in vitro fertilization programs.77 In that study, data from 700,000 in vitro fertilization cycles performed in 120 assisted reproduction clinics were used.77 These results were in direct contrast with the negative impact of obesity on male fertility and the results of in vitro fertilization widely reported in experimental studies conducted in humans and animals.78–85
The information discussed in this article suggests that oviductal and uterine microenvironments are affected by overnutrition, which makes the notion that the harmful effects of overfeeding may be directly exerted on the reproductive tract likely. Additional studies (i.e. metabolomic and proteomic) are needed for in-depth characterization of the effects of overnutrition on the reproductive tract.
ConclusionsLong-term overnutrition normally leads to obesity. Obesity may affect reproductive capacity not only through anovulation, but also through changes in the ovary and reproductive tract (Fig. 2). Changes in ovarian follicular growth occurring in obesity are very likely to result in ovulation of oocytes with a deficient developmental capacity. Information derived from animal models suggests that oviductal and uterine microenvironments are also affected by overnutrition. These changes may affect the embryo in the preimplantation phase, with a negative impact on the chances of a successful pregnancy. Further experimental studies are however required for in-depth characterization (i.e. metabolic, molecular, and cellular effects) of the effects of overfeeding during the periconceptional period. Such studies would provide information relevant for the development of preventive strategies that increase the probability of successful pregnancy in obese individuals. This type of research is however difficult to perform in humans, because studies on this subject are usually conducted on couples with fertility problems. Animal models are therefore needed in order to perform such detailed research.
Conflicts of interestThe author states that he has no conflicts of interest.
Please cite this article as: Velázquez MA. Impacto de la sobrenutrición materna sobre el periodo de periconcepción. Endocrinol Nutr. 2015;62:246–253.