Graves-Basedow disease is the most common cause of hyperthyroidism. As is well known, this is an autoimmune disease in which TSI antibodies (thyroid-stimulating immunoglobulins) stimulate thyroid hormone synthesis and release, provided an adequate amount of iodine is available. The condition may be triggered by an episode of emotional stress, infection, pregnancy or delivery, or an increased iodine intake at a given time.
In clinical history, drugs such as amiodarone, the use of iodinated contrast media, or iodinated salt consumption are usually suspected, but a more comprehensive search is sometimes required.
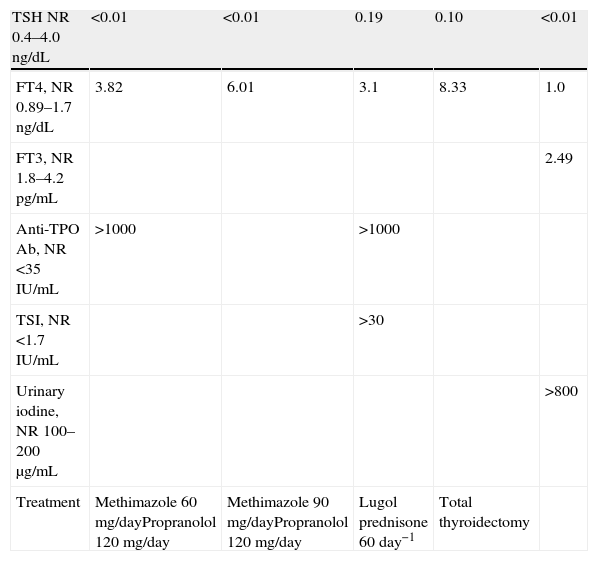
A 42-year-old female patient with an unremarkable history attended her general practitioner reporting a loss of 12kg in weight and nervousness during the previous months. Thyroid function tests were requested with the following results: TSH<0.01 (NR, 0.4–4.0ng/dL); FT4: 3.28 (NR, 0.89–1.76ng/dL); and anti-TPO antibodies >1000 (NR, <35IU/mL). Treatment was started with methimazole 5mg every 8h, which was uptitrated to 60mg daily due to lack of improvement, and propranolol 40mg every 8h. The results of the last laboratory tests on the above treatment were TSH <0.01 and FT4 6.01ng/dL. The dosage was increased to 90mg daily and the patient was referred to the endocrinology department for work-up.
The patient reported progressive weight loss for four months, as well as intense daily palpitations, nervousness, and insomnia. She was taking iodinated salt and regularly used a hair dye, but this did not contain iodine. No drug intake or recent history of use of iodinated contrast media was found. The use of creams of other topical substances with high iodine contents was not reported. She was advised to stop using iodinated salt.
Physical examination findings included: 72.8kg of weight (86kg previously); body mass index, 27kg/m2; blood pressure, 145/80mmHg; and heart rate of 100bpm. The patient had a bright gaze with minimal lid retraction. No exophthalmos was found. Palpation revealed an elastic, nontender grade II thyroid gland with no nodules. The examination was otherwise normal.
A thyroid scan showed strong and uniform hyper-uptake. A thyroid ultrasound examination was consistent with diffuse thyroid disease.
The results of laboratory tests performed three weeks after the maximal methimazole dose included: TSH 0.19, FT4 3.1, anti-TPO antibodies >1000IU/m; and TSI antibodies >30 (NR, <1.7IU/mL).
Tests performed after six weeks on maximum methimazole doses with proven treatment compliance found the following results: TSH 0.1 and FT4 8.33ng/dL.
Based on a diagnosis of primary hyperthyroidism due to Graves-Basedow disease, and because of the lack of response to drug treatment and severe clinical symptoms, surgical treatment was decided upon.
It was also decided that treatment compliance during admission for preparation for total thyroidectomy should be monitored. A ward examination revealed skin lesions possibly due to scratches. The patient was asked to bring with her all drugs and antiseptics she used at home. She had given no importance to the fact that she had a cat and used povidone iodine almost daily to heal the wounds caused by scratches. A test for urinary iodine was requested.
Lugol, 8 drops every 6h, and prednisone 60mg every 24h were subsequently added to the treatment. This achieved control of thyroid hormones before total thyroidectomy, which was uneventful. Urinary iodine level, reported after surgery, was >800μg/mL (NR, 100–200) (Table 1).
Changes over time in thyroid function tests.
| TSH NR 0.4–4.0ng/dL | <0.01 | <0.01 | 0.19 | 0.10 | <0.01 |
| FT4, NR 0.89–1.7ng/dL | 3.82 | 6.01 | 3.1 | 8.33 | 1.0 |
| FT3, NR 1.8–4.2pg/mL | 2.49 | ||||
| Anti-TPO Ab, NR <35IU/mL | >1000 | >1000 | |||
| TSI, NR <1.7IU/mL | >30 | ||||
| Urinary iodine, NR 100–200μg/mL | >800 | ||||
| Treatment | Methimazole 60mg/dayPropranolol 120mg/day | Methimazole 90mg/dayPropranolol 120mg/day | Lugol prednisone 60day−1 | Total thyroidectomy |
NR: normal range.
The daily iodine requirements for thyroid hormone synthesis are 150μg. Mean urinary iodine level in the US is 14.5μg/dL,1 but up to 50–60μg/dL in other countries with a higher intake, such as Iceland.
The thyroid gland has a regulation mechanism that maintains normal function even in the presence of excess iodine. Although the release of T4 and T3 may decrease in the first 48h due to decreased iodine organification (the Wolff-Chaikoff effect), hormone hyperproduction may eventually result (Jod-Basedow). It is estimated that the amount of iodine ingested below which thyroid function is not affected is 500μg/day.
In areas with an endemic iodine deficiency, hyperthyroidism induced by excess iodine intake may occur in patients with a multinodular thyroid gland, autonomous nodules, or latent Graves-Basedow disease due to increased thyroid hormone production and release.2 Its incidence is 1.7%.
Areas with adequate iodine intake have a low incidence of hyperthyroidism induced by excess iodine intake.
Euthyroid patients with some prior episode of postpartum thyroiditis, type 2 amiodarone-induced thyrotoxicosis, or interferon-induced thyroid dysfunction are more susceptible to develop hyperthyroidism due to excess iodine intake (up to 20%), as are patients with multinodular thyroid, autonomous nodules, or diffuse goiter.3,4 In the latter, the prevalence ranges from 3.5% to 21% depending on iodine exposure.
Iodine intake may set the course in patients with Graves-Basedow disease, because a slight increase in dietary iodine results in a greater frequency of hyperthyroidism and a decreased efficacy of antithyroid treatment.5 In addition, in iodine-deficient areas, the response to antithyroid agents is better and lower doses are required for hormone control.6
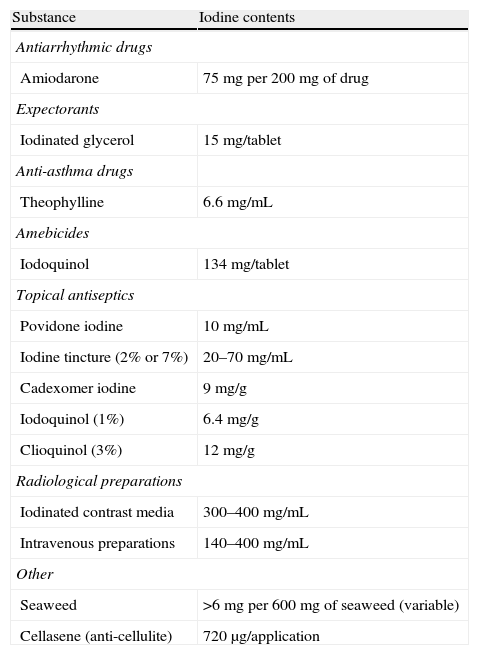
It is therefore essential to consider the potential factors leading to excess iodine intake when faced with difficult to control Graves-Basedow disease (Table 2).
Iodine contents in different substances.
| Substance | Iodine contents |
| Antiarrhythmic drugs | |
| Amiodarone | 75mg per 200mg of drug |
| Expectorants | |
| Iodinated glycerol | 15mg/tablet |
| Anti-asthma drugs | |
| Theophylline | 6.6mg/mL |
| Amebicides | |
| Iodoquinol | 134mg/tablet |
| Topical antiseptics | |
| Povidone iodine | 10mg/mL |
| Iodine tincture (2% or 7%) | 20–70mg/mL |
| Cadexomer iodine | 9mg/g |
| Iodoquinol (1%) | 6.4mg/g |
| Clioquinol (3%) | 12mg/g |
| Radiological preparations | |
| Iodinated contrast media | 300–400mg/mL |
| Intravenous preparations | 140–400mg/mL |
| Other | |
| Seaweed | >6mg per 600mg of seaweed (variable) |
| Cellasene (anti-cellulite) | 720μg/application |
In the case of our patient, the course of hyperthyroidism led us to decide upon a definitive treatment. The clinical condition of the patient and the course of events prevented us from detecting excess iodine intake before surgery or a potential improvement after the removal of povidone iodine, but a more satisfactory response to drug treatment could have been expected in the absence of excess iodine intake.
Please cite this article as: Civantos S, et al. Implicación del aporte excesivo del yodo en la enfermedad de Graves-Basedow. Endocrinol Nutr. 2013;60:273–5.