Diabetes mellitus has been treated as a chronic disease, but may now be considered as a condition where remission is possible. In this regard, we would define remission as the achievement of glycemic goals below the diabetic range in the absence of active pharmacological or surgical treatments. A distinction may be made between partial remission (glycosylated hemoglobin [HbA1c] less than 6.5% and fasting blood glucose ranging from 100 to 125mg/dL) and complete remission (HbA1c in the normal range and fasting blood glucose less than 100mg/dL for longer than one year without active drug treatment).
For remission of type 2 diabetes mellitus (T2DM) there are two possibilities: metabolic/bariatric surgery or an effort by the patient to implement lifestyle changes consisting of weight loss and physical exercise.1
Treatment goals for comorbidities (high blood pressure or dyslipidemia) should be the same in a patient who achieves partial or complete remission as in a patient with diabetes, although less strict goals could be considered when remission lasts longer than five years.1
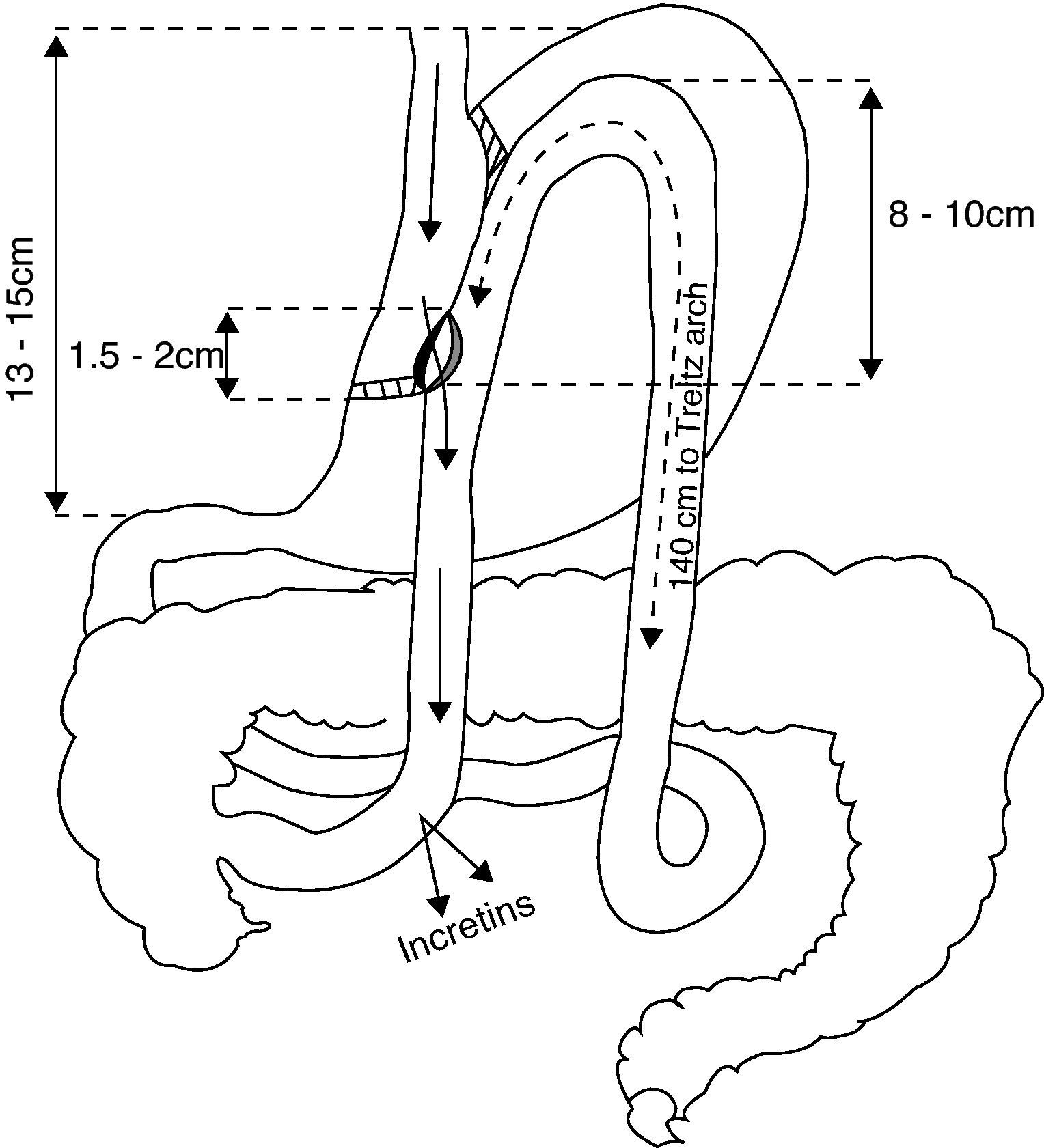
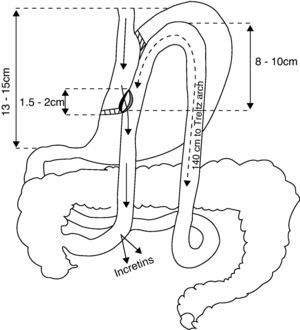
We report here three patients who underwent laparoscopic gastric bypass with side-to-side gastrojejunal anastomosis (Fig. 1).2 All of them were monitored at our outpatient clinic, but we were not involved in the surgical indication.
The first patient was a 70-year-old male with a 22-year history of T2DM, treated with insulin for the previous 12 years. Patient history also included two acute myocardial infarctions, hypercholesterolemia, diabetic polyneuropathy, and erectile dysfunction. Blood glucose control was variable. Before surgery, he had a body mass index (BMI) of 33.5kg/m2, HbA1c of 6.4% and C-peptide level of 0.88ng/mL with no hypoglycemia, and was being treated with a mixture of 30% insulin aspart and NPH at doses of 34-12-26, atorvastatin, ezetimibe, valsartan, and ASA. Laparoscopic gastric bypass with anastomosis was performed, and three months later the patient had BMI 27.3kg/m2 and HbA1c 7.7% on insulin glargine 20 units daily and metformin 850mg daily.
The second patient was a 58-year-old male with a 14-year history of T2DM, treated with insulin for the previous 5 years. His personal history included hypercholesterolemia, high blood pressure, cough secondary to ACEIs, heart failure attributed to the use of rosiglitazone, and hemodynamic angina due to a hypertensive crisis. His course showed variable blood glucose control. Before surgery, the patient had BMI 33.8kg/m2, C-peptide 0.90ng/mL (1.1–5ng/mL) and HbA1c 7.9% and was being treated with 25% insulin lispro mix (24 units at breakfast and 30 units at dinner), doxazosin, atorvastatin, and ASA. The patient returned to the clinic without any treatment for one month after undergoing gastric bypass and the following values were found: BMI 30.4kg/m2, BP 186/91mmHg, and HbA1c 7%. After missing visits to the clinic, the patient was found to have an HbA1c level of 10% four months after surgery.
The third patient was a 56-year-old male initially diagnosed with T2DM 11 years earlier after experiencing diabetic ketoacidosis. He was put on variable treatment with insulin therapy and oral antidiabetics. One year later he was found to be positive for anti-GAD, and the diagnosis was changed to LADA. He had been on insulin therapy for the past 8 years and attended visits irregularly. Five years before his HbA1c was 9.2%, and his prior insulin scheme was replaced by a basal-bolus scheme with insulin glargine and aspart. The patient discontinued visits to the clinic. Gastric bypass was performed with laboratory values of 0.06ng/mL of C-peptide and 8.6% of HbA1c. One month after surgery, the patient was admitted to hospital with severe diabetic ketoacidosis after discontinuing insulin treatment.
The three reported cases may be seen as a failure based on the results achieved after metabolic surgery, related to inadequate indication of the procedure. This was essentially due to the time lapse since disease onset and the need for insulin treatment in the first two patients, and to the complete absence of insulin secretion due to LADA in the third patient.
It is indispensable that we consider the pathophysiology of diabetes mellitus and the potential mechanisms by which a patient may be amenable to the remission of disease after the performance of these surgical procedures, and thus establish indications for this type of treatment.
The rationale for metabolic surgery is that an improvement of diabetes mellitus sometimes occurs within days or weeks of standard bariatric surgery, before weight loss has occurred.3 The potential mechanisms involved in diabetes improvement independently of weight loss would be those related to the effect of an increase in regulatory incretins (GLP-1, GIP), which would increase C-peptide levels, thus improving the pancreatic function.4
The Diabetes Surgery Summit Conference, attended by endocrinologists, diabetologists, surgeons, and basic researchers, established consensus criteria.5 They agreed on the need to promote randomized clinical trials to establish both the safety and efficacy of gastrointestinal surgery as a treatment for T2DM and selection criteria beyond body mass index. Only 66% of the qualified participants in the summit agreed that treatment should be performed in patients with BMI values less than 35kg/m2.
In a 2008 study conducted in 20 patients with T2DM duration of 5.3 years (2–8) and mean BMI of 27.1kg/m2 (25–30), mean HbA1c levels decreased from 8.8% (7.5–10.2) to 6.8% (5.8–7.9) six months after laparoscopic duodenojejunal exclusion. C-peptide levels also improved.6 Although patients with a shorter duration of diabetes and better C-peptide levels had a better postoperative course, most of them did not achieve the abovementioned disease remission criteria.
A database with 109 participants with BMI ranging from 30 to 35kg/m2 on drug treatment for diabetes and who underwent a gastric bypass was subsequently published.7 An interesting aspect of this study was that a comorbidity scale was established by which 61% of patients with SCORE 2 (controlled on oral medication) were discontinued medication after 24 months of follow-up. On the other hand, 50% of patients with SCORE 4 (controlled on oral medication and insulin) no longer needed treatment for disease control. However, biochemical markers were not used.
Based on the current evidence, the pathophysiological mechanisms other than weight that would lead to diabetes remission, or at least improvement, are not sufficiently clear. Moreover, clear-cut indications for surgery cannot be established in these types of patients, at least in those with normal weight or BMI less than 35kg/m2. By contrast, it appears that pancreatic reserve markers and a short time lapse since the onset of T2DM could suggest better candidates for metabolic surgery and so improve disease remission results. Randomized clinical trials, preferably with the participation of endocrinologists and general surgeons, will be needed in this regard.
The question also arises as to whether the achieving of disease remission goals would have an influence on the improvement of the complications derived from diabetes (retinopathy, nephropathy, neuropathy, or macrovascular complications).
Please cite this article as: Peñalver Talavera D, et al. Cirugía metabólica: a propósito de 3 casos. Endocrinol Nutr. 2012;59(8):516–19.