The ability to predict recurrence of pituitary adenoma (PA) after surgery may be helpful to determine follow-up frequency and the need for adjuvant treatment. The purpose of this study was to assess the prognostic capacity of pituitary tumor transforming gene (PTTG), insulin-like growth factor 1 receptor (IGF1R), and Ki-67.
Materials and methodsIn this retrospective study, the normalized copy number (NCN) of PTIG and IGF1R mRNA was measured using RT-PCR, and the Ki-67 index was measured by immunohistochemistry in 46 PA samples. Clinical data, histological subtype, and radiographic characteristics were collected to assess associations between variables and tumor behavior. Progression of tumor remnants and its association to markers was also studied in 14 patients with no adjuvant treatment after surgery followed up for 46±36 months.
ResultsExtrasellar tumors had a lower PTTG expression as compared to sellar tumors (0.065 [1st–3rd quartile: 0.000–0.089] NCN vs. 0.135 [0.105–0.159] NCN, p=0.04). IGF1R expression changed depending on histological subtype (p=0.014), and was greater in tumor with remnant growth greater than 20% during follow-up (10.69±3.84 NCN vs. 5.44±3.55 NCN, p=0.014).
ConclusionsOur results suggest that the IGF1R is a more helpful molecular marker than PTTG in PA management. Ki-67 showed no association to tumor behavior. However, the potential of these markers should be established in future studies with standardized methods and on larger samples.
La capacidad de predecir recurrencia en los adenomas hipofisarios (AH) tras la cirugía puede ser útil para determinar la frecuencia de seguimiento y la necesidad de tratamientos adyuvantes. El objetivo del presente estudio fue valorar la capacidad pronóstica de gen transformador de tumores hipofisarios (pituitary tumor transforming gene [PTTG]), del receptor del factor de crecimiento insulinoide 1 (insulin-like growth factor 1 receptor [IGF1R]) y de Ki-67.
Material y métodosEn este estudio retrospectivo determinamos el número de copias normalizadas de ARNm (Cnn) de PTTG e IGF1R mediante RT-PCR y el índice Ki-67 mediante inmunohistoquímica en 46 muestras de AH. Los datos clínicos, el subtipo histológico y las características radiológicas se recogieron para determinar asociaciones entre las variables y el comportamiento tumoral. Además, estudiamos la progresión de los restos tumorales y su asociación con los marcadores en 14 pacientes sin tratamiento adyuvante posquirúrgico seguidos durante 46±36 meses.
ResultadosLos tumores extraselares mostraron una expresión de PTTG menor que los intraselares (0,065 [1.er-3.er cuartil: 0,000-0,089] Cnn frente a 0,135 [0,105–0,159] Cnn, p=0,04). La expresión de IGF1R varió en función del subtipo histológico (p=0,014), siendo mayor en los tumores que presentaron crecimiento de los restos mayor del 20% durante el seguimiento (10,69±3,84 Cnn frente a 5,44±3,55 Cnn, p=0,014).
ConclusionesNuestros resultados indican que IGF1R, en mayor medida que PTTG, es un marcador molecular útil en el manejo de los AH. Ki-67 no mostró asociación con el comportamiento tumoral. Sin embargo, el potencial de estos marcadores debe ser establecido en futuros estudios con una metodología estandarizada y una muestra mayor.
Pituitary adenomas (PA) constitute 10–25% of intracranial neoplasms. They are almost always benign, but some show aggressive behavior with local invasion and recurrences. Several sporadic mutations of oncogenes and tumor-suppressor genes have been found in PA, but none has been found to be a reliable marker of poor outcome.1,2 In clinical practice, Ki-67 antigen expression is frequently used as a prognostic indicator because it has been associated with proliferative potential and invasiveness of several human malignancies, but it has shown discordant results in pituitary adenomas.3,4
Thus, there is an increasing interest in finding specific prognostic markers. Pituitary tumor transforming gene (PTTG) encodes a protein that functions like securin and transcription factor.5,6 It is involved in the cell cycle regulation and may induce cellular proliferation and aneuploidy.6,7 Overexpression of PTTG has been described in several neoplasms including PA.8–10
IGF1R is a tyrosine kinase receptor responsible for mediating IGF-I signaling, which plays a critical role in normal growth and has been associated with the early stages of tumor establishment. IGF1R stimulates the PI3K/Akt and MAPK pathways,11 resulting in cell proliferation and apoptosis alteration. Its overexpression has been documented in many human malignancies12 but it is not yet well studied in PA.
The aim of this study is to examine whether PTTG and IGF1R expression or Ki-67 index may have prognostic implications on pituitary adenomas.
2Materials and methods2.1SubjectsThis is a retrospective study performed at Hospital General Universitario de Alicante including patients who underwent surgery for PA between 1995 and 2008. We included cases that had complete hormonal tests and magnetic resonance imaging (MRI) upon diagnosis. Minimum follow-up needed was 3 months after surgery, with the exceptions of patients who died prior to this (2 patients died of postoperative complications and one due to cardiac arrest). Clinical data were retrieved from medical files. Tumor tissue samples were obtained from the formalin-fixed and paraffin-embedded remaining fragments of pathological diagnosis. Overall, 46 patients were included in the study. This study was approved by the Ethics Committee of the Hospital General Universitario de Alicante.
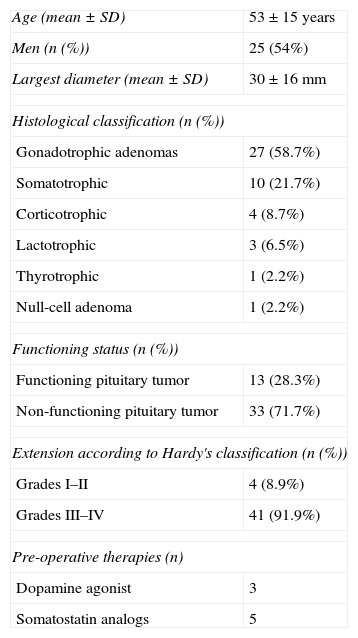
Histological classification was performed according to WHO 2004 classification criteria (Table 1). We also assessed functional status, considering functioning pituitary tumors (FPA), those associated with hypersecretion of prolactin, ACTH, GH, TSH and specific hormone-related syndrome. Lack of a hormone related syndrome, whether associated with biologically inactive hypersecretion or not, was defined as a non-functioning pituitary adenoma (NFPA). Invasiveness was defined as optic chiasm compression or invasion of surrounding structures like cavernous or sphenoidal sinus. Other baseline characteristics are summarized in Table 1.
Patient characteristics.
| Age (mean±SD) | 53±15 years |
| Men (n (%)) | 25 (54%) |
| Largest diameter (mean±SD) | 30±16mm |
| Histological classification (n (%)) | |
| Gonadotrophic adenomas | 27 (58.7%) |
| Somatotrophic | 10 (21.7%) |
| Corticotrophic | 4 (8.7%) |
| Lactotrophic | 3 (6.5%) |
| Thyrotrophic | 1 (2.2%) |
| Null-cell adenoma | 1 (2.2%) |
| Functioning status (n (%)) | |
| Functioning pituitary tumor | 13 (28.3%) |
| Non-functioning pituitary tumor | 33 (71.7%) |
| Extension according to Hardy's classification (n (%)) | |
| Grades I–II | 4 (8.9%) |
| Grades III–IV | 41 (91.9%) |
| Pre-operative therapies (n) | |
| Dopamine agonist | 3 |
| Somatostatin analogs | 5 |
As this is a retrospective study, we considered patient follow-up to be the time between surgery and the last visit to the Endocrinology Unit. Following surgery, fourteen patients received adjuvant therapies: DA (1), SSa (9) or ketoconazole (2). Thirteen patients received radiotherapy and 12 underwent a second surgery during the follow-up period due to incomplete resection after first surgery (only one of the tumor remnants experienced significant growth before radiotherapy). Then, we studied the progression of PA after surgery in patients who did not receive early postoperative treatment. Fourteen patients, with a 46±36 (mean±SD) months of follow-up, were included in this analysis. Aggressiveness was defined as the growth of tumor remnants greater than 20% in any of the tumor diameters measured. We reviewed all the MRI scans performed during the follow-up period in order to establish the time of progression.
2.2Immunohistochemical study: Ki-67 indexImmunohistochemical study was performed as previously described.13 The number of positive cells defines the Ki-67 index. We applied a Ki-67 index cut-off values of 3% associated with high proliferative activity.
2.3RNA source, total RNA extraction and reverse transcriptionWe used 2 cylindrical cores (0.6mm-thick) from pre-selected tumor areas of FFPE tissues, to extract and isolate total RNA by using RNAeasy FFPE Kit (QIAgen) according to the protocol and working under manufacturer-recommended conditions. We assessed the quality and quantity of RNA extracted from this using the NanoDrop. Reverse transcription of 2μg of RNA was performed in 20μL reaction volumes with random hexamer primers, with High Capacity cDNA Reverse Transcription Kit (Applied Biosystems®) and stored at −20°C until used.
2.4Real time Q-PCRTo select the control genes, we analyzed the expression of 32 housekeeping genes in three pools consisting of 10 sample mixes each, on a commercial endogenous plate based on TaqMan assay technologies (Applied Biosystems). The results, when analyzed with GeNorm software, showed that the most stable genes were GAPDH and YWHAZ.
To analyze the gene expression of PTTG and IGF1R genes, we chose the TaqMan Assays for each gene under manufacturer-recommended conditions. We constructed standard curves that were used to check that the amplification efficiency of the housekeeping genes selected and the target genes were similar (with a difference less than 10%) and estimated the number of copies of mRNA. Specific primers were designed to amplify the cDNA fragments detected by each TaqMan assay with Primer-BLAST. The primers chosen were subject to the Basic Local Alignment Search Tool (BLAST) database, which searches to find any similarities in sequence. We used commercial brain total RNA (Ambion®) as a template, which was transcribed together with adenoma samples. PCR amplification was performed in a 25μL reaction volume containing 2.5mM MgCl2, 0.5μM of each primer, 1 unit of KAPA2G Robust HotStar Taq polymerase (KAPABiosystems®) and 25ng of cDNA. PCR reactions were amplified for 35 cycles with an annealing temperature of 55°C. The amplified fragments were purified by Qiaquick Purification Kit (Qiagen®). We confirmed the specificity of the primers and obtained the concentration of the amplified fragments using the 2100 Bioanalyzer (Agilent®). Dilutions were made for standards such as 109, 1010, 1011, 1012, 1013 and 1014 copies of mRNA, calculated from the total ng of the PCR product (according to the formula MW=[number of nucleotides×607.4]+157.9). Real-time PCRs of serial dilutions were performed in triplicate in 12.5μL reaction volumes containing 6.25μL of TaqMan Gene Expression Master Mix (Applied Biosystems®), 0.625μL of TaqMan Gene Expression Assay, and 2.5μL of template in 7500 Real Time PCR System (Applied Biosystems) and were analyzed using SDS software (Applied Biosystems®). The R2 values for all standard curves generated were higher than 0.99 and all the efficiencies of the TaqMan assays were higher than 90%. We made an RT-minus control using Total Human DNA (Roche) to ensure that the TaqMan assays did not detect amplification of genomic DNA.
Analysis of the samples was performed in accordance with the same protocol. We included two dilutions of standard curves and no template control (NTC) on all QPCR plates to ensure that efficiencies were maintained and that they were free from contamination. All analyses were performed in duplicate. The results from each plate were only validated when the NTC was undetectable and the dCt maintained a standard deviation below 0.33 between duplicates and between both dilutions of standard curves and their initial results.
Since both housekeeping genes were highly correlated (Spearman rank correlation coefficient: r=0.82; p<0.000), all the results were normalized to YWHAZ. We used standard curves to estimate the copy number normalized (Cnn) of PTTG and IGF1R.
2.5Statistical analysesData were tested for statistical significance using SPSS 11.0 software (SAS Institute®). Associations between the molecular assays and the clinical–pathological features were calculated. IGF1R showed a normal distribution, whereas the rest of molecular and IHC variables showed a non-normal distribution (Kolmogorov–Smirnov test). The Student's t-test and the Mann Whitney U-test were used for comparison between two groups of parametric and nonparametric data, respectively. The Chi-square test followed by Fisher's exact test, where appropriate, was used to identify correlations between categorical parameters. The Kruskal–Wallis test was used for comparison of more than two categories of non-parametric data. Receiver operating characteristics (ROC) curves were used to establish the optimal PTTG and IGF1R cut-offs for distinguishing between invasive and/or aggressive pituitary adenomas. Kaplan–Meier survival analysis and the log-rank test were used to compare progression-free interval curves. The effect of a variable was estimated by the odds ratio (OR) of the model and a 95% confidence interval (95% CI) was calculated for each OR. Data are shown as mean±standard deviation (normal distribution) or median and quartiles (non-normal distribution). p values<0.05 were considered statistically significant.
3ResultsWe investigated a series of 46 patients with pituitary adenomas. Tumors were classified according to WHO 2004. Table 1 summarizes the clinical–pathological features.
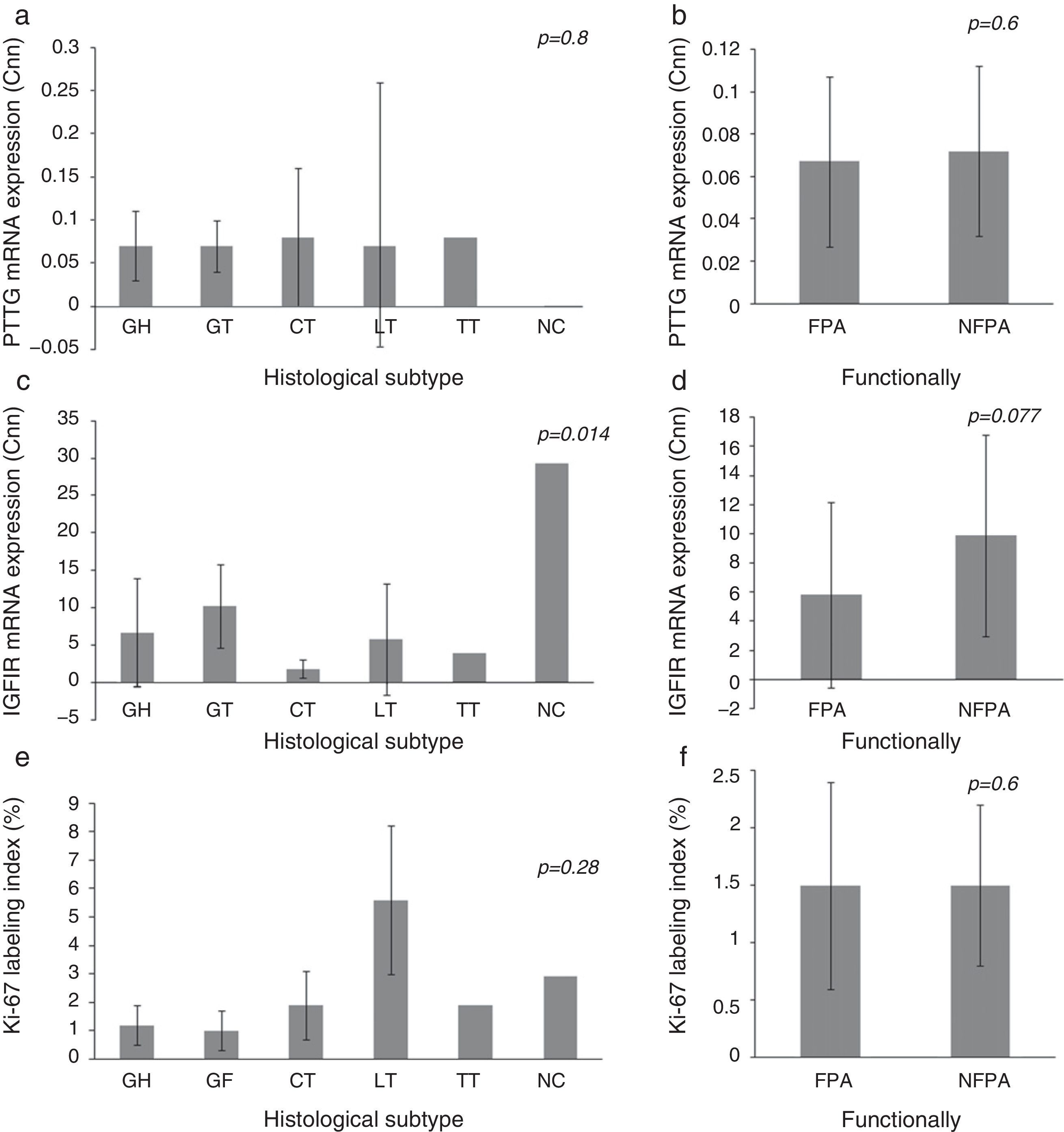
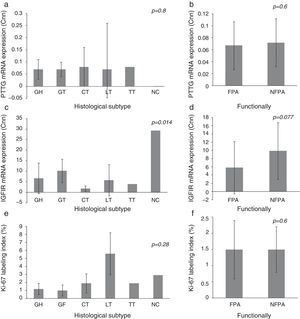
3.1Expression of IGF1R mRNA, PTTG mRNA and Ki-67 index on histological subtypes of PAIGF1R expression differed between histological subtypes (p=0.014) and had its greatest expression in NC, followed by GT. There were no statistically significant differences in PTTG mRNA expression or Ki-67 index between different histological subtypes, although the Ki-67 index was higher in LT. We did not find significant difference in the expression of molecular variables between FPA and NFPA, although there was a trend toward more IGF1R expression in the NFPA subgroup (5.82±6.43 Cnn vs. 9.85±6.91 Cnn, p=0.077) (Fig. 1).
Pituitary tumor transforming gene (PTTG) and insulin-like growth factor I receptor (IGF1R) Cnn expression and Ki-67 index (%) according to histological subtypes (a, c, e) and functional status (b, d, f). Data reported as mean±S.E.M. Non-parametric Kuskal–Wallis and U-Mann Whitney tests were used with p values of 0.05 or less being considered significant. Abbreviations: GH, somatotrophic adenomas; GT, gonadotrophic adenomas; CT, corticotrophic adenomas; LT, lactotrophic adenomas; TT, Thyrotrophic adenomas; NC, null-cell adenomas; FPA, functioning pituitary adenoma; NFPA, non-functioning pituitary adenoma; Cnn, copy number normalized.
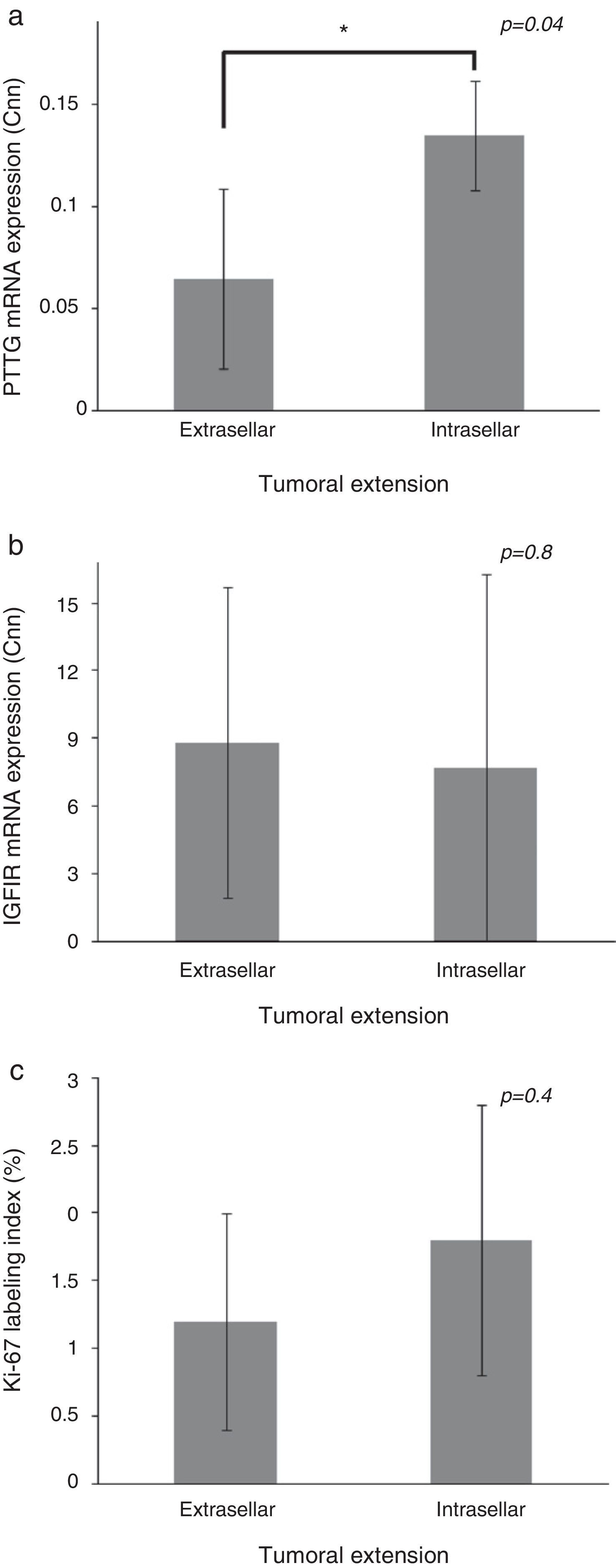
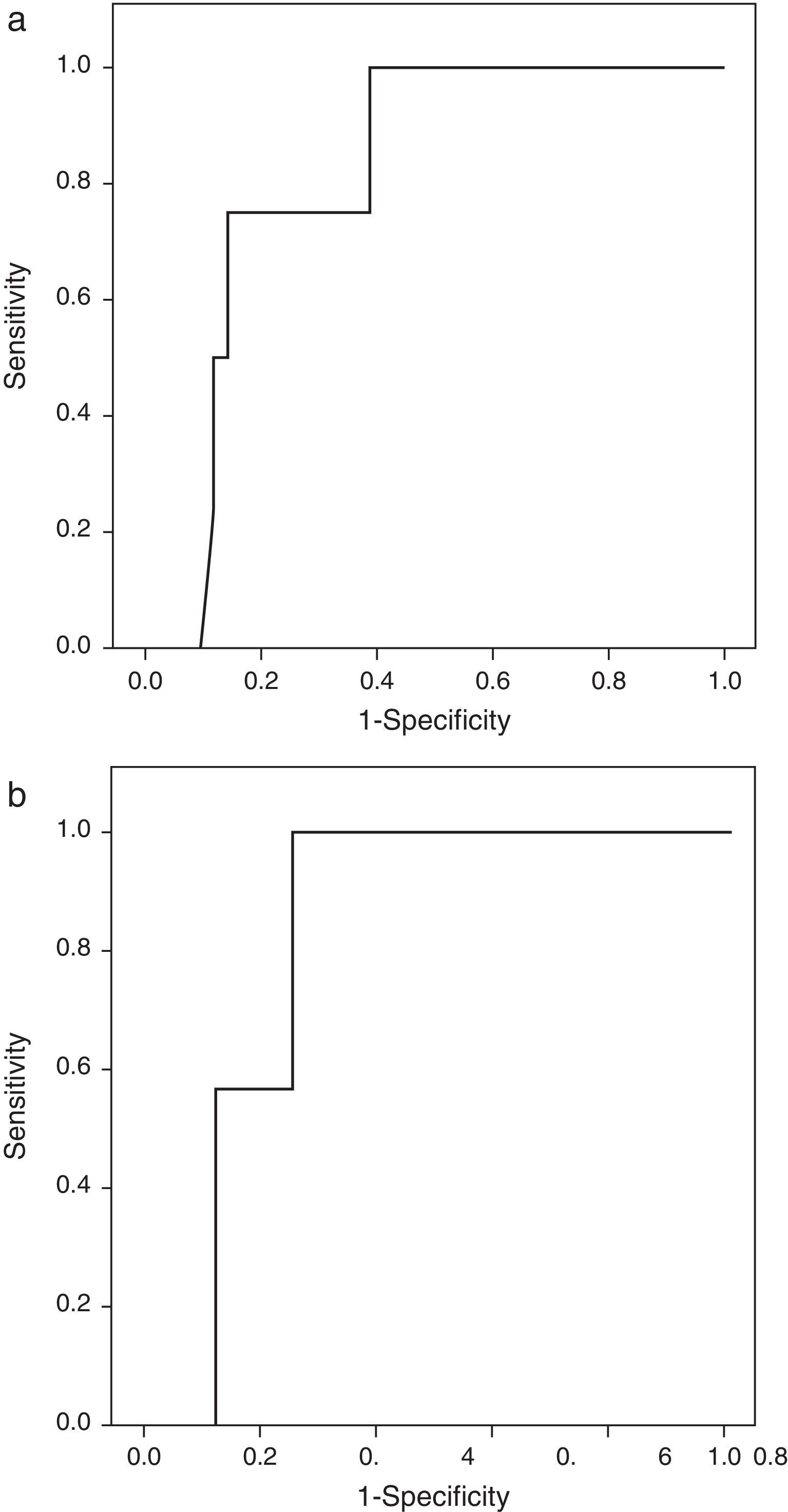
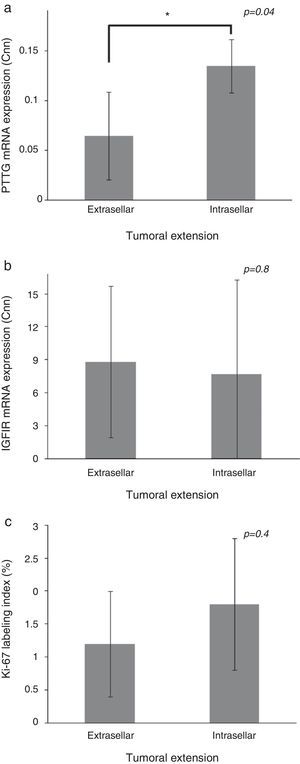
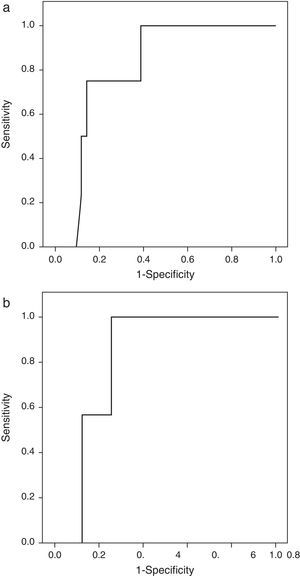
Patients with extrasellar extension showed lower PTTG mRNA expression (0.065 [P25–P75: 0.000–0.089] Cnn vs. 0.135 [0.105–0.159] Cnn, p=0.04) (Fig. 2). ROC analysis identified a significant cut-off point for PTTG predicting extrasellar extension (Fig. 3a). A cut-off of 0.129 PTTG mRNA Cnn or less predicted extrasellar extension with a sensitivity of 75% and specificity of 85%. Cases with less than 0.129 Cnn of PTTG mRNA had 17.5-fold risk of extrasellar extension (95% CI, 1.6–197.4, p=0.02). There were no statistically significant differences in clinical variables (age, sex, tumor size, histological classification, functioning status, extension according to Hardy's classification or pre-operative therapies), IGF1R mRNA expression or the Ki-67 index (Fig. 2).
Comparison of pituitary tumor transforming gene (PTTG) (a) and insulin-like growth factor I receptor (IGF1R) (b) Cnn expression and Ki-67 index (%) (c) between intrasellar and extrasellar tumors. Data reported as mean±S.E.M. Non-parametric U-Mann Whitney test was used with p values of 0.05 or less being considered significant. Abbreviations: Cnn, copy number normalized.
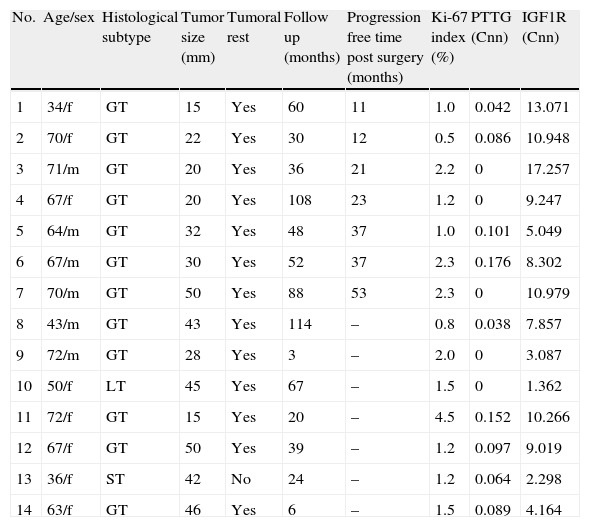
We studied the tumoral behavior of 14 patients who did not receive adjuvant postoperative therapies (Table 2). During follow-up period, 7 tumors grew more than 20% on at least one of the diameters measured. The average time free of progression was 26±15 months. All tumors had extrasellar extension at diagnosis.
Clinical characteristics of patients without adjuvant therapies.
| No. | Age/sex | Histological subtype | Tumor size (mm) | Tumoral rest | Follow up (months) | Progression free time post surgery (months) | Ki-67 index (%) | PTTG (Cnn) | IGF1R (Cnn) |
| 1 | 34/f | GT | 15 | Yes | 60 | 11 | 1.0 | 0.042 | 13.071 |
| 2 | 70/f | GT | 22 | Yes | 30 | 12 | 0.5 | 0.086 | 10.948 |
| 3 | 71/m | GT | 20 | Yes | 36 | 21 | 2.2 | 0 | 17.257 |
| 4 | 67/f | GT | 20 | Yes | 108 | 23 | 1.2 | 0 | 9.247 |
| 5 | 64/m | GT | 32 | Yes | 48 | 37 | 1.0 | 0.101 | 5.049 |
| 6 | 67/m | GT | 30 | Yes | 52 | 37 | 2.3 | 0.176 | 8.302 |
| 7 | 70/m | GT | 50 | Yes | 88 | 53 | 2.3 | 0 | 10.979 |
| 8 | 43/m | GT | 43 | Yes | 114 | – | 0.8 | 0.038 | 7.857 |
| 9 | 72/m | GT | 28 | Yes | 3 | – | 2.0 | 0 | 3.087 |
| 10 | 50/f | LT | 45 | Yes | 67 | – | 1.5 | 0 | 1.362 |
| 11 | 72/f | GT | 15 | Yes | 20 | – | 4.5 | 0.152 | 10.266 |
| 12 | 67/f | GT | 50 | Yes | 39 | – | 1.2 | 0.097 | 9.019 |
| 13 | 36/f | ST | 42 | No | 24 | – | 1.2 | 0.064 | 2.298 |
| 14 | 63/f | GT | 46 | Yes | 6 | – | 1.5 | 0.089 | 4.164 |
m, male; f, female.
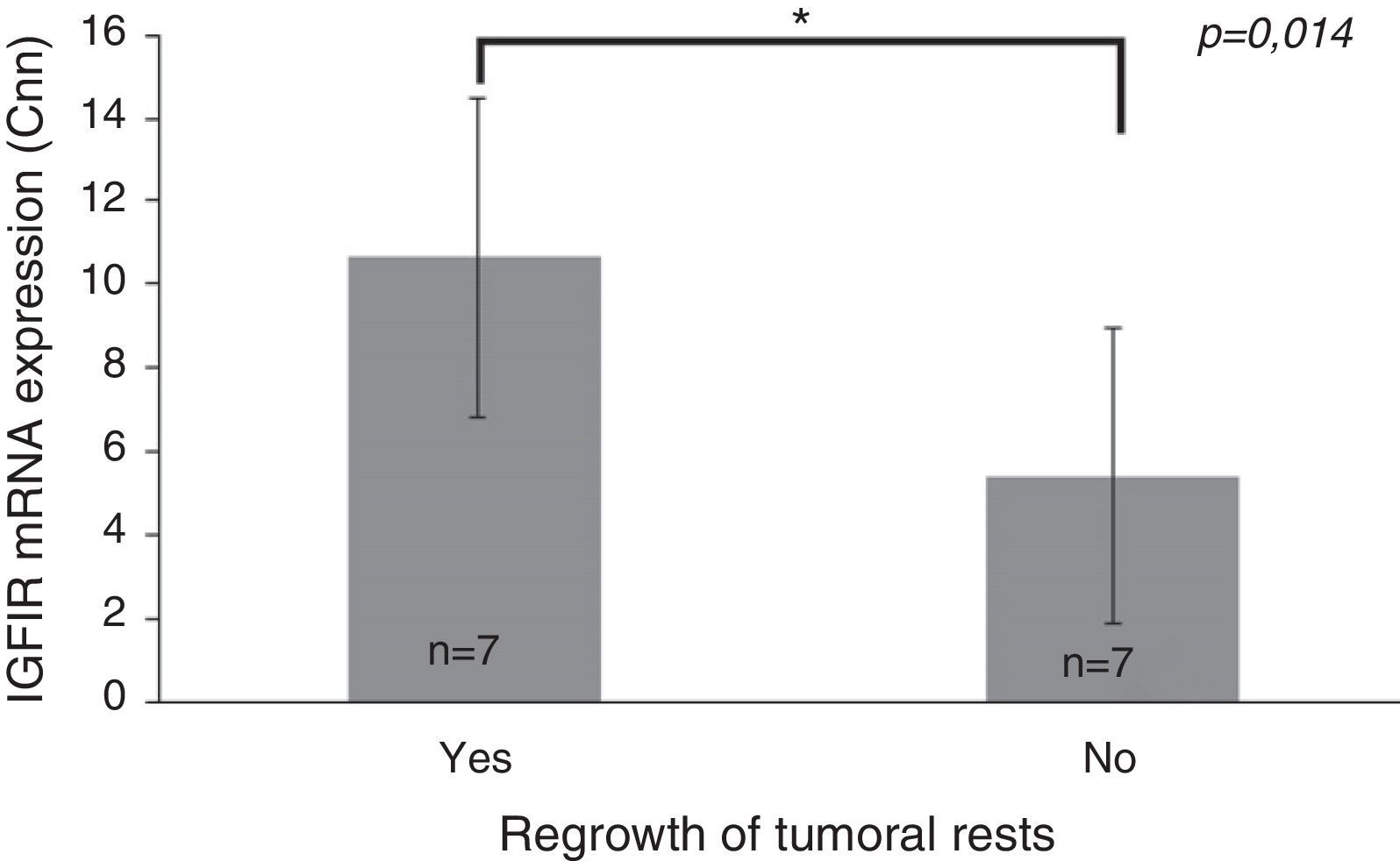
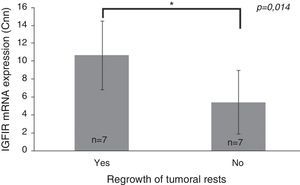
Only IGF1R mRNA Cnn showed an association with progression (10.69±3.84 Cnn vs. 5.44±3.55 Cnn, p=0.014) (Fig. 4). Patients with more than 4.61 Cnn of IGF1R (25th percentile) had 2.3 (95% CI 0.99–5.49, p=0.07) times more risk of regrowth during the follow-up period. Through ROC curve analysis, this cut-off had a sensitivity of 100% and specificity of 75% (p=0.010) (Fig. 3b). However, PTTG and Ki-67 index were not associated with tumor progression. Similarly, neither age nor sex showed association with progression.
Differences between insulin-like growth factor I receptor (IGF1R) Cnn expression according to tumoral progression during the follow-up in tumors without post-surgical treatment. Data reported as mean±S.E.M. Non-parametric U-Mann Whitney test was used with p values of 0.05 or less being considered significant. Abbreviations: Cnn, copy number normalized.
On survival analysis, although without statistical significance, tumors without PTTG expression or with IGF1R expression less than 9 Cnn (50th percentile) had more time free of progression (32.3±10.3 vs. 24.3±7.36 months, p>0.05; and 37.0±0 vs. 24.0±7.6 months, p>0.05, respectively).
4DiscussionSome PAs demonstrate aggressive behavior, which lends to invasiveness of adjacent local structures, or have early regrowth (recurrence) following surgery. Thus, it is very important to identify the tumors that will become aggressive early on, in order to intensify the follow-up or to consider adjuvant therapies.
Nowadays, the pathological index most used to predict the aggressive behavior of PAs has been the Ki-67 index. However, it is a semi-quantitative measurement that is highly dependent on the observer and the prognostic capability of Ki-67 index in PA has been less predictive than in others tumors.4
In our series, we did not find any difference in Ki-67 index between invasive and non-invasive tumors. Nevertheless, higher Ki-67 index was found in LT than in other histological subtypes (5.6% [3.6–7.8]) and the three LT were classified as invasive. Several authors have demonstrated that Ki-67 index is significantly higher in FPA than in NFPA.4 When we analyzed LT, CT, TT and ST together vs. GT and the one NC in our series, we did not find any significant differences. This discrepancy could be attributed to the fact that 5/10 ST in our series had been treated previously with SSa and one of the three LT had received dopamine agonist. It has been suggested that preoperative treatment of ST and LT adenomas may decrease Ki-67 levels, resulting in values similar to those of NFPA.14 In any case, several studies have failed to show a significant difference among adenomas of varying hormonal subtypes or functional status.4
Different criteria used to define extension or invasiveness could explain the controversy on literature regarding the relationship between the invasiveness and Ki-67 index levels. Some authors consider invasion to be when there is histological evidence of dural invasion; but others, as us, base it on the radiologic findings on preoperative MRI. The discrepancy was greater when the invasion assessment was based on MRI imaging criteria. As an example, Wolfsberger et al.15 found a correlation between Ki-67 index and radiographic extension (1.9% in invasive adenomas vs. 1.7% in non-invasive adenomas) in adenomas 3cm or less in diameter only. We did not find any significant difference in Ki-67 levels between invasive vs. non-invasive tumors (1.2 vs. 1.8), and we were unable to find a cut-off for Ki-67 index to predict the aggressive behavior of PAs that showed recurrence. Thus, we agree with Wolfsberger et al.15 having emphasized the limitation of relying solely on Ki-67 to predict tumor behavior. Moreover, only some studies demonstrated a correlation between Ki-67 and recurrence. These discrepancies may be attributable in part to variations in the definition of recurrence and the duration of follow-up. In our case, we considered aggressiveness as growth of primary tumor remnants and this situation has been found to be correlated to recurrence.16 With respect to follow up, we did not find a significant correlation between Ki-67 index and recurrence after 46±36 months, similar to the findings of Scheithauer and Dubois that had follow-up over 9 years.1,17 Thus, it appears that Ki-67 alone is a poor predictor of tumor recurrence, which demonstrates the need to search for new markers of tumor behavior.
Many investigators have searched for other biological markers that could help with decision making. In recent years, IGF1R overexpression has been described in many types of cancer.11,12 However, the data on IGF1R in pituitary tumor pathology almost all come exclusively from animal pituitaries or established cell line derived from the pituitary and only a few authors have investigated this gene in human PA.
Otsuka et al.18 analyzed the mRNA expression of IGF1R, IGF-I, IGF-II and other growth factors in six LT, six ST and ten GT adenomas. They found lower IGF1R and IGF-I expression in ST than in LT and GT. In 2003, Kola et al.19 studied a series of 18 ST and other PAs and found that IGF1R mRNA levels were significantly lower in ST and tended to be lower in CT than in normal pituitary tissue. In our series, we also found different gene expression among histological subtypes, with the greatest expression in the null-cell PA, followed by GT, and the lowest expression in CT (Fig. 1), similar to the Otsuka et al. report.18 To date, no author has studied the relationship between IGF1R expression and the clinical behavior of PA, although it has become apparent that it is associated with adverse prognosis in some type of tumors.20 IGF1R overexpression has been associated with protecting cancer cells and giving the tumor capabilities such as anchorage-independent growth and surviving the process of detachment required for metastasis.21,22
In our series, we did not find a relationship between IGF1R overexpression and the invasiveness of PA, possibly due to our arbitrary definition of invasion. However, we found a positive association between expression of the IGF1R gene and aggressiveness of the PA. In fact, IGF1R expression greater than 4.61 Cnn may distinguish tumors with significant growth during follow up with a sensitivity of 100% and specificity of 75%.
There are no references in literature with which to compare our results; however, they are consistent with studies carried out on the molecular biology of PAs. Thus, loss of E-cadherin expression or function and its nuclear translocation are well recognized as causing cell detachment and have been linked to pituitary tumor invasion.23 The IGF1R pathway activation can result in the disruption of β-catenin/E-cadherin complexes, favoring the relocation of IRS-1/β-catenin complexes to the nucleus to enhance β-catenin transcriptional activity24. Similarly, tumor cell motility and invasive potential are influenced by IGF-induced secretion of metalloproteinase, enzymes that can degrade and reorganize the extracellular matrix (ECM) and whose overexpression is usually associated with tumor invasion and metastasis.25 Therefore, our results are consistent with the hypothesis that IGF1R expression can be related to the outcome of PA.
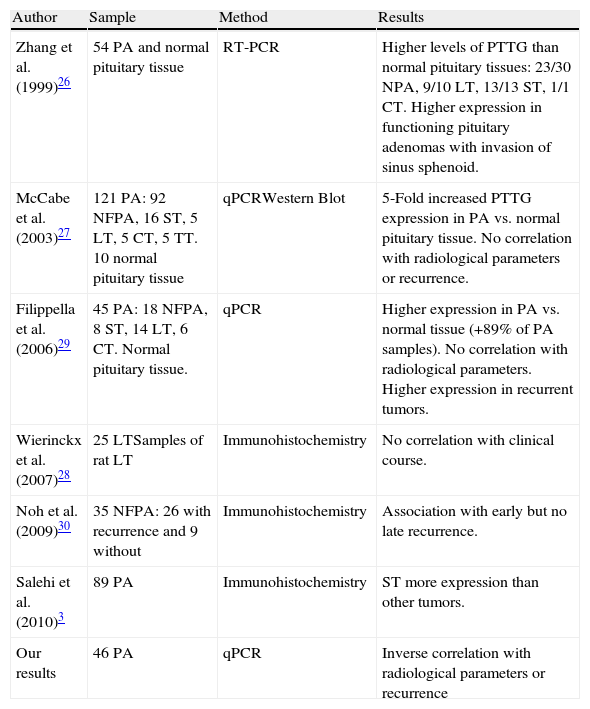
Another protein widely studied in regard to its relationship with tumorigenesis is PTTG. All studies that analyze normal pituitary tissue find higher PTTG expression in pituitary adenomas than in normal tissue. However, data from literature show a high discrepancy between the results of different studies of PTTG expression as marker of invasive and aggressive behavior of PA.10Table 3 shows the conflicting views existing among different authors regarding the influence of PTTG on the behavior of a PA. Only Zhang et al.26 find a correlation between its levels and the pre-operative extension of the FPA but not in NFPA.
Description of studies on relationship between pituitary tumor transforming gene (PTTG) expression and pituitary adenoma behavior.
| Author | Sample | Method | Results |
| Zhang et al. (1999)26 | 54 PA and normal pituitary tissue | RT-PCR | Higher levels of PTTG than normal pituitary tissues: 23/30 NPA, 9/10 LT, 13/13 ST, 1/1 CT. Higher expression in functioning pituitary adenomas with invasion of sinus sphenoid. |
| McCabe et al. (2003)27 | 121 PA: 92 NFPA, 16 ST, 5 LT, 5 CT, 5 TT. 10 normal pituitary tissue | qPCRWestern Blot | 5-Fold increased PTTG expression in PA vs. normal pituitary tissue. No correlation with radiological parameters or recurrence. |
| Filippella et al. (2006)29 | 45 PA: 18 NFPA, 8 ST, 14 LT, 6 CT. Normal pituitary tissue. | qPCR | Higher expression in PA vs. normal tissue (+89% of PA samples). No correlation with radiological parameters. Higher expression in recurrent tumors. |
| Wierinckx et al. (2007)28 | 25 LTSamples of rat LT | Immunohistochemistry | No correlation with clinical course. |
| Noh et al. (2009)30 | 35 NFPA: 26 with recurrence and 9 without | Immunohistochemistry | Association with early but no late recurrence. |
| Salehi et al. (2010)3 | 89 PA | Immunohistochemistry | ST more expression than other tumors. |
| Our results | 46 PA | qPCR | Inverse correlation with radiological parameters or recurrence |
Abbreviations: PA, pituitary adenoma; FPA, functioning pituitary tumors; NFPA, non-functioning pituitary tumors; GT, gonadotrophic adenomas; ST, somatotrophic adenoma; CT, corticotrophic adenoma; LT, prolactinoma; TT, thyrotrophic adenoma; NC, null-cell adenoma.
Our results show lower PTTG expression in invasive tumors than in non-invasive tumors. When we studied only clinically functional adenomas, we found significantly higher PTTG expression in intrasellar vs. extrasellar tumors; however, we considered all types of extrasellar extension of PA, whereas Zhang et al.26 found higher PTTG expression only in sphenoid sinus invasion. In addition, Zhang et al.26 measured PTTG using a densitometry method on electrophoresis results after Reverse Transcription PCR, a semi-quantitative technique, while we used the current gold standard, real-time PCR with TaqMan® technology, which is a more sensitive and specific quantitative technique. On the whole, given the overall methodological differences, we feel that the results are not comparable. Although our results may suggest the role of PTTG as protective in FPA, the low number of patients studied in our series influences the strength of our results. Nevertheless, PTTG has demonstrated in vitro and in vivo activity as protooncogen, inducing cell proliferation and tumor growth, as well as securin, inhibiting proliferation; so this double function could explain the lower PTTG expression, with low securin activity, found in extrasellar tumors compared with intrasellar ones.9 After all, we agree with other authors27,28 that PTTG cannot be considered a biological marker of invasiveness on PA.
With respect to aggressiveness, Filippella et al.29 in their series found that a PTTG score of 3.3% clearly distinguished between recurrent and non-recurrent pituitary adenomas with a sensitivity of 60% and specificity of 76% (ROC curve method); and Noh et al.30 found early, no later than one year after surgery, recurrence in tumors with the highest levels of PTTG. Paradoxically, to Filippella et al.29 the fact that 18/45 patients in their series had a follow-up less than 1 year could dilute the relationship between PTTG and recurrence; therefore, they analyzed the results for 27 patients with more than 1 year of follow up separately. Thus, once again, the results from literature are conflicting. Moreover, McCabe et al.27 using similar technology as us, real-time PCR, in the largest published PA series, did not observe a significant association between mRNA expression and the presence of recurrent tumor growth, although the authors did not report length of the follow up.
We demonstrated progression in 7/14 patients who did not receive post-operative adjuvant treatments during the follow-up (46±36 months). To minimize bias, we performed an analysis of recurrence-free survival (Kaplan Meier). On the whole, we did not find a correlation between PTTG expression and aggressiveness; however, when we separated our sample by PTTG expression, the patients without PTTG expression show a tendency to have more time free of recurrence, although not reaching statistical significance.
Our main limitation was that we studied retrospectively a small cohort which could affect the strength of our results and our sample was a very heterogeneous group of tumors (different endocrine and non-endocrine types, in some cases only one representative of an adenoma type). Moreover, you should note that a comparison of the various tumor types in FPA is difficult since some types (e.g. prolactinomas) are treated surgically only when medical treatment fails. This selects for the more aggressive tumors and could lead to a selection bias. Furthermore, it has not been possible to use the relative quantification by qPCR using ddCT method because it was not possible to obtain normal tissue. Nevertheless, we have adapted our protocol to estimate the number of copies of the different genes’ transcripts and we have only compared the behavior of these genes among different subtypes of PA or among the different variables studied.
In conclusion, we have found that IGF1R is associated with progression of tumor rests and it can be new marker in PA outcome. In regard to PTTG, our data do not suggest clearly prognostic implications in PA according with some previous studies. Further work involving large series of patients and a prospective analysis needs to be performed in order to clarify the potential prognostic role of new biomarkers in clinical practice.
5Conflict of interestThis work was supported by Pfizer Grant.