To provide practical recommendations for evaluation and management of hypoglycemia in patients with diabetes mellitus.
ParticipantsMembers of the Diabetes Mellitus Working Group of the Spanish Society of Endocrinology and Nutrition.
MethodsRecommendations were formulated according to the Grading of Recommendations, Assessment, Development, and Evaluation system to describe both the strength of recommendations and the quality of evidence. A systematic search was made in MEDLINE (PubMed). Papers in English and Spanish with publication date before 15 February 2013 were included. For recommendations about drugs only those approved by the European Medicines Agency were included. After formulation of recommendations, they were discussed by the Working Group.
ConclusionsThe document provides evidence-based practical recommendations for evaluation and management of hypoglycemia in patients with diabetes mellitus.
Proporcionar unas recomendaciones prácticas para la evaluación y el manejo de la hipoglucemia en pacientes con diabetes mellitus.
ParticipantesMiembros del Grupo de Trabajo de Diabetes Mellitus de la Sociedad Española de Endocrinología y Nutrición (SEEN).
MétodosLas recomendaciones se formularon de acuerdo al sistema Grading of Recommendations, Assessment, Development, and Evaluation para establecer tanto la fuerza de las recomendaciones como el grado de evidencia. Se realizó una búsqueda sistemática en MEDLINE (PubMed) de la evidencia disponible para cada tema, y se revisaron artículos escritos en inglés y castellano con fecha de inclusión hasta el 15 de febrero de 2013. Para las recomendaciones acerca del uso de fármacos, se consideraron tratamientos aprobados por la Agencia Europea de Medicamentos con esa misma fecha. Tras la formulación de las recomendaciones estas se discutieron conjuntamente por el Grupo de trabajo.
ConclusionesEl documento establece unas recomendaciones prácticas basadas en la evidencia acerca de la evaluación y manejo de la hipoglucemia en pacientes con diabetes mellitus.
Hypoglycemia induced by glucose lowering treatment is one of the main factors preventing the achievement of adequate metabolic control, which is essential for the prevention of complications in patients with diabetes mellitus (DM).1,2 Hypoglycemia is associated with excess morbidity and mortality, increases the costs associated with the care of DM, and involves a loss of productivity in the patients affected.3–6
The Diabetes Mellitus Working of the Spanish Society of Endocrinology and Nutrition considered the evaluation and management of hypoglycemia in patients with DM to be a priority area for the development of clinical practice guidelines, and prepared these evidence-based recommendations.
Preparation of evidence-based clinical practice guidelinesThe following recommendations were made based on the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) system to establish the strength of recommendations and the level of evidence.7 In terms of strength, a distinction is made between strong recommendations, expressed as “We recommend” and number 1, and weak recommendations, expressed as “We suggest” and number 2. The quality of the evidence is expressed by symbols: ⊕○○○ indicates very low evidence; ⊕⊕○○, low evidence; ⊕⊕⊕○, moderate evidence; and ⊕⊕⊕⊕, high evidence. After each recommendation, the evidence supporting it is provided.
A systematic search was made in MEDLINE (PubMed) of the evidence available for each subject, and articles written in English and Spanish with an inclusion date up to 15 February 2013 were reviewed. For drug use recommendations, treatments approved by the European Medicines Agency until that same date were considered. Once the recommendations were formulated, they were jointly discussed by the Working Group.
Definition and classification of hypoglycemiaRecommendations- -
We recommend the evaluation of the presence and severity of symptomatic and asymptomatic hypoglycemia at each visit by patients with type 1 (T1DM) and type 2 diabetes mellitus (T2DM) who are at risk of hypoglycemia (1⊕⊕⊕○).
- -
We suggest that patients with DM be warned about the potential occurrence of hypoglycemia when glucose levels decrease rapidly or are less than 70mg/dL in capillary blood glucose self-monitoring (CBGSM) (2⊕○○○).
In patients with DM, hypoglycemia is defined as any episode of abnormally low plasma glucose levels (with or without symptoms) in which the patient is exposed to harm.8,9 The value below which hypoglycemia is defined in patients with DM, 70mg/dL, is higher than that used to diagnose hypoglycemia in non-diabetic patients (less than 55mg/dL) and is not free from controversy.10–12 Its definition is based on the normal glycemic threshold for counterregulatory hormone secretion.9 In practice, hypoglycemia is classified based on its clinical consequences.8
Severe hypoglycemiaThis is hyperglycemia where the help of another person who administers carbohydrates (CHs), glucagon, or other measures is required for recovery. Although no blood glucose measurement is available, neurological recovery attributable to the restoration of normal glucose levels is considered as adequate evidence.
Symptomatic documented hypoglycemiaThe typical symptoms of hypoglycemia are associated with a plasma glucose measurement of less than 70mg/dL.
Asymptomatic hypoglycemiaA plasma glucose measurement of less than 70mg/dL with no associated symptoms.
Probable symptomatic hypoglycemiaThe typical symptoms of hypoglycemia not associated with plasma glucose measurement, but presumably caused by plasma glucose levels of less than 70mg/dL.
Relative hypoglycemiaA patient with DM shows the typical symptoms of hypoglycemia and interprets them as indicative of hypoglycemia, but the plasma glucose level measured is higher than 70mg/dL. This reflects the fact that patients with poor glycemic control may experience symptoms of hypoglycemia with plasma glucose levels higher than 70mg/dL.
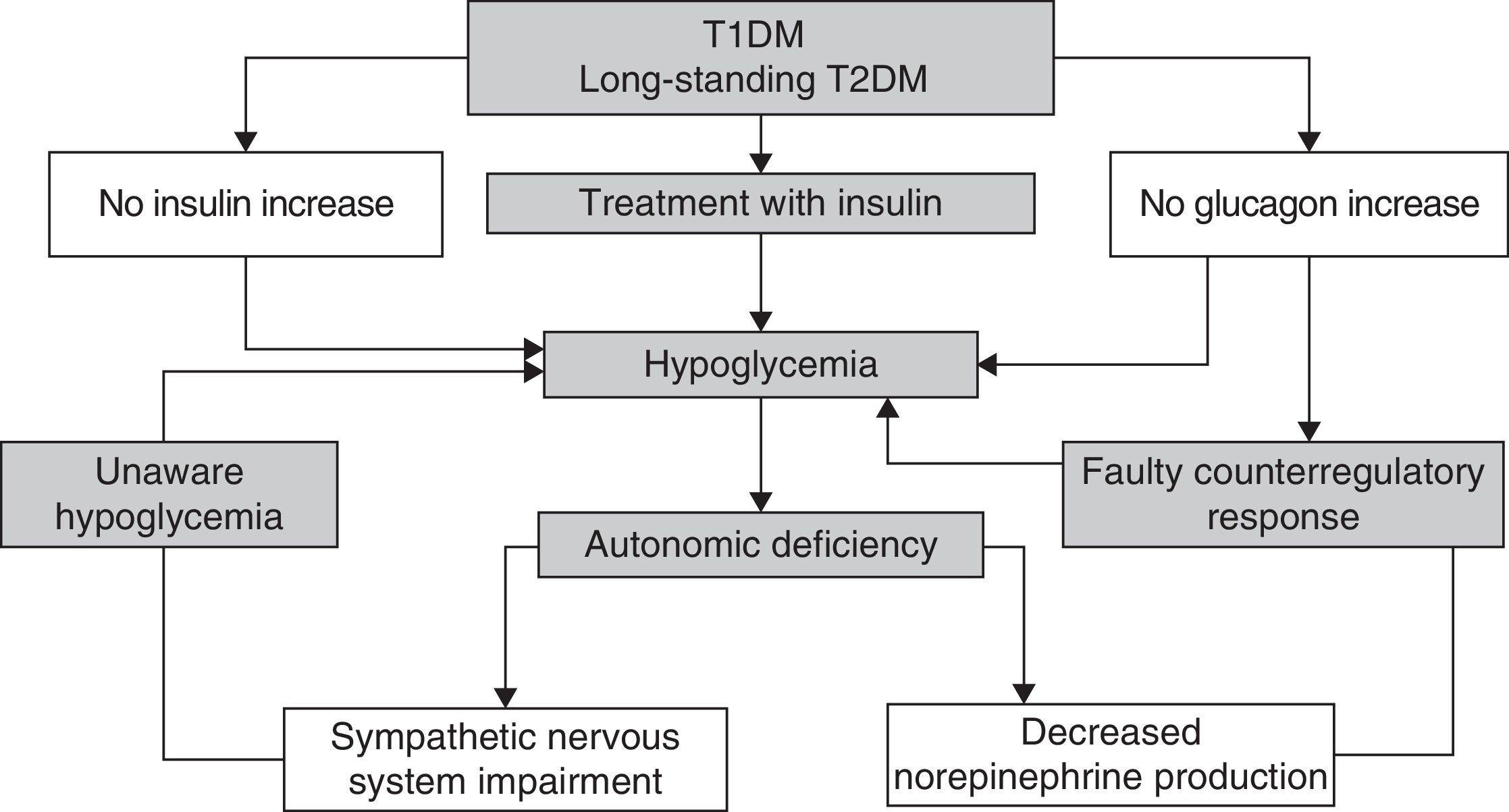
Counterregulatory responseUnder physiological conditions, the initial response to hypoglycemia is the inhibition of endogenous insulin secretion,13 which does not occur in patients with T1DM or in many patients with T2DM. In addition, there are a number of counterregulatory hormones whose actions result in increased plasma glucose levels: glucagon, norepinephrine, growth hormone (GH), and cortisol.13 There is also a neurogenic response to hypoglycemia started by neural glucose sensors at the peripheral and central level, mediated by different neurotransmitters responsible for some of the neurological symptoms of hypoglycemia.14
Blood glucose thresholds triggering the different counterregulatory mechanisms vary,14 and are also modified by different pathophysiological situations occurring in DM. Increased glucagon levels are, together with the inhibition of insulin secretion, the first line of response to hypoglycemia, stimulating glycogenolysis and indirectly promoting gluconeogenesis. Epinephrine secretion plays a secondary role in counterregulation after glucagon, but becomes important when glucagon secretion is deficient. Epinephrine actions include the stimulation of glycogenolysis and gluconeogenesis in the liver (and also in the kidney), and decreased peripheral glucose utilization.13 In addition, it also directly inhibits insulin secretion by beta cells.
Hyperglycemia also induces a response of the sympathetic and parasympathetic autonomous nervous system, which exerts direct counterregulatory actions by neural action at peripheral level, limiting insulin secretion and stimulating the secretion of counterregulatory hormones. These include GH and ACTH, whose secretion is stimulated through the hypothalamus. Cortisol and GH increases have a hyperglycemic effect starting at 2–3h, and their actions result in increased glucose production in the liver and decreased peripheral glucose utilization.
Hypoglycemia in type 1 diabetesRecommendations- -
We recommend the prevention of hypoglycemia through the achievement of an adequate balance between insulin dose, intake, and physical activity, and an active search for hypoglycemia using CBGSM, particularly in patients who have had DM for more than five years (1⊕⊕⊕⊕).
- -
We recommend the assessment of treatment with continuous subcutaneous insulin infusion (CSII) pumps in patients with T1DM and frequent hypoglycemia (either severe or not) (1⊕⊕⊕⊕).
Iatrogenic hypoglycemia is associated with insulin treatment in T1DM and is one of the main factors preventing the achievement of glucose control goals. It is estimated that blood glucose may be less than 50mg/dL in up to 10% of the lifetime of patients with T1DM. On average, these patients experience two episodes per week of symptomatic hypoglycemia and an annual episode of severe hypoglycemia.13 In addition, it is estimated that one out of every 25 patients with T1DM will die from iatrogenic hypoglycemia.15
The Diabetes Control and Complications Trial found an incidence of severe iatrogenic hypoglycemia of 62 episodes per 100 patient/year.16 More recently, however, the United Kingdom Hypoglycemia Study Group has found an incidence of severe hypoglycemia in patients with T1DM treated with insulin for less than 10 years of 110 episodes per 100 patient/year,17 similar to that reported by the Stockholm Diabetes Intervention Study,18 and an incidence of 320 episodes of severe hypoglycemia per 100 patient/year when patients with T1DM treated with insulin for longer than 15 years were included.17 On the other hand, in a prospective observational study including 7067 patients with T1DM, the incidence was 300 episodes of hypoglycemia per 100 patient/year.19
Various meta-analyses have shown that treatment with CSII decreases up to fourfold the number of severe hypoglycemic episodes; this reduction is greater in patients with a higher number of prior severe hyperglycemia episodes.20 A 50%–70% decrease in the total number of hypoglycemic episodes has also been shown.21
Hypoglycemia in type 2 diabetesRecommendations- -
We recommend, as a priority objective in T2DM, the prevention of hypoglycemia because of its association with a greater probability of treatment discontinuation, increased costs, and impaired quality of life (1⊕⊕⊕⊕).
In patients with T2DM treated with insulin and/or oral antidiabetics in the US, the estimated frequency of visits to a medical center for hypoglycemia of any type was 0.054 per patient/year. Hypoglycemia was associated with a greater probability of treatment discontinuation and increased healthcare costs, either or not related to DM.22
The incidence of hypoglycemia in patients with T2DM reported by the different studies is variable. In a large observational study, the incidence of severe hypoglycemia was 11.8 episodes per 100 patient/year, similar to that found in patients with T1DM in the same study.23 The frequency of non-severe hypoglycemia is very difficult to estimate. In a retrospective study24 of 14,357 patients treated with oral antidiabetics and/or insulin, 11% of the patients had already experienced at least one episode of “significant” hypoglycemia in the previous 12 months. In patients with T2DM, the incidence of mild and severe hypoglycemia in patients treated with insulin for longer than five years was similar to that seen in patients with T1DM, while patients with T2DM treated with insulin for less than two years had an incidence of hypoglycemia similar to that seen during treatment with sulfonylureas (SUs) and lower than reported in patients with T1DM.17
Hypoglycemia and cardiovascular diseaseRecommendations- -
We recommend that, in T2DM, hypoglycemia be considered a factor associated with cardiovascular disease (CVD) (1⊕⊕○○), and severe hypoglycemia as a factor associated with overall mortality (1⊕⊕⊕○).
- -
We suggest that severe hypoglycemia in T1DM should not be considered as a factor associated with the occurrence of CVD (2⊕⊕○○).
In the Action in Diabetes and Vascular Disease (ADVANCE) study,5 severe hypoglycemia was associated with a significant increase in overall mortality risk (adjusted hazard ratio [HR]: 3.30; confidence interval [CI]: 2.31–4.72). The Action to Control Cardiovascular Risk in Diabetes study25 also showed a significant association between severe hypoglycemia and death from any cause in both the intensive group (HR: 1.41, CI: 1.03–1.93) and the conventional group (HR: 2.30, CI: 146–3.65), with no relationship between severe and/or asymptomatic hypoglycemia and mortality.26
Observational studies also support the relationship between hypoglycemia and overall mortality, except for one where no relationship was found between overall mortality and severe hypoglycemia of any type after four years of follow-up.27 In one study, self-reported severe hypoglycemia was associated with a HR for five-year mortality of 3.4 (CI: 1.5–7.4) as compared to those who had reported non-severe or no hypoglycemia28; in another study, adjusted HR was 2.48 (CI: 1.41–4.38) for overall mortality in patients with severe or non-severe hypoglycemia.29 A systematic review of the Veterans’ Health Administration concluded that adequate evidence is available to establish the association between severe hypoglycemia and overall mortality in the long term, but not in the short term.30
The relationship between hypoglycemia and cardiovascular death is less well established, and no adequate data are available to confirm or rule out this association. The only data available came from the post hoc analysis of the ADVANCE study,5 which showed a significant relationship between severe hypoglycemia and cardiovascular death (HR: 3.78, CI: 2.34–6.11). No temporal relationship was found between severe hypoglycemia and mortality, and no dose-response relationship was seen either. This led the authors to wonder whether severe hypoglycemia played a causative role in mortality or was merely a marker of the risk or vulnerability for the occurrence of complications.
As regards the relationship of hypoglycemia to overall CVD occurrence in T2DM, the ADVANCE5 study specifically showed the association of severe hypoglycemia with CVD (HR: 2.88, CI: 2.01–4.12). The remaining evidence comes from observational studies, one of which showed a significant relationship between CVD and any hypoglycemia in patients with T2DM after four years of follow-up (HR: 2, CI: 1.63–2.44)26; another study reported an association between hypoglycemia and CVD (OR: 1.79, CI: 1.69–1.89),31 and a third study reported that severe hypoglycemia accounted for a HR of 2.09 (CI: 1.63–2.67) of associated CVD.29 In addition, a cross-sectional study noted an increased incidence of symptomatic hypoglycemia in patients with T2DM and CVD as compared to those with no CVD (OR: 3.73, CI: 1.31–10.65).32 Again, these data did not allow a causal relationship to be established.
In subjects with T1DM, the large clinical trials conducted16,33 showed no greater total of cardiovascular mortality or CVD in the intensive treatment group, which also had a higher incidence of hypoglycemia. A Spanish retrospective study found an association between a history of severe hypoglycemia and the development of CVD, which disappeared after adjusting for age and DM duration.34 Finally, in the EURODIAB Prospective Complications Study,35 including 2181 patients with T1DM followed up for longer than seven years, the incidence of CVD was associated with the frequency of severe hypoglycemia.
Hypoglycemia and risk of fracture in patients with diabetes mellitusRecommendations- -
We recommend that hypoglycemic episodes be considered as being associated with an increased risk of fracture in patients with DM (1⊕⊕○○).
- -
We suggest therapeutic strategies aimed at preventing falls related to hypoglycemia and improving bone health in patients with DM and fragility (2⊕○○○).
DM, falls, and fractures are common conditions in elderly people. Falls are related to damage to different organs and particularly to fractures.36 Patients with T2DM have various risk fractures for falls and fractures: advanced age, decreased physical activity, peripheral and autonomic neuropathy, decreased vision, lower limb amputation, vitamin D deficiency, and glitazone therapy.37 Antidiabetic drugs may influence fracture risk, including the risks of hypoglycemia and falls, by various mechanisms.
A recent retrospective, observational study assessed the association between hypoglycemia and fall-related fractures in a cohort of 361,210 patients with T2DM.38 In this study, patients with episodes of hypoglycemia had a 70% greater risk of fall-related fractures as compared to patients with no hypoglycemia (OR=1.70; 95% CI: 1.58–1.53). Hip and vertebral fractures were most common. In another observational case and control study in a cohort of 1945 patients with T2DM followed up for more than four years, insulin treatment was significantly associated with fractures in males (OR=3.20; 95% CI: 1.32–7.74).39 The remaining evidence comes from case series and the descriptions of isolated cases in patients with T1DM and T2DM, mostly associated with seizures.
Hypoglycemia and physical exerciseRecommendations- -
We recommend CBGSM by all patients with T1DM before, during, and after the practice of physical exercise (1⊕○○○).
- -
We recommend that the rapid insulin bolus be reduced before exercise (when exercise is performed 90–120min after the bolus) and/or that CH intake be modified to prevent hypoglycemia (1⊕⊕○○).
- -
We recommend the intake of CHs before exercise is started if the blood glucose level is less than 100mg/dL, and after exercise depending on the blood glucose level (1⊕⊕○○).
- -
We suggest that insulin be reduced after exercise and/or that CHs be taken after exercise (2⊕⊕○○) to prevent hypoglycemia after physical activity.
- -
In patients with T2DM treated with SUs or repaglinide and/or insulin, we recommend that blood glucose be checked before physical exercise (1⊕○○○) and that drug treatment be adjusted to prevent hypoglycemia associated with exercise (1⊕⊕○○).
In patients with T1DM, capillary blood glucose should be measured before, during, soon afterwards, and several hours after the end of exercise.40 If exercise lasts longer than 30min and is performed 2–3h after the injection of rapid-acting insulin analogs or 4–6h after regular insulin, a 50%–90% reduction in insulin dose should be considered depending on the intensity and duration of the planned exercise.41,42 An extra amount of CH (10–20g) should also be ingested if pre-exercise blood glucose levels are less than 100mg/dL.43,44 Intake of glucose (fortified drinks or food) at a rate of 1g/kg/h improves performance and decreases the risk of hypoglycemia.45
The hypoglycemic effect is greater in the 60–90min subsequent to physical activity,46 but persists for 6–15h after its completion.47 Counterregulatory response is also decreased, which may affect the perception of hypoglycemia.48 A 10-s sprint at maximum intensity before or after exercise49 decreases the risk of hypoglycemia immediately after exercise by inducing a catecholamine response. An intake of 5mg/kg of caffeine before exercise decreases hypoglycemia during and after exercise.50 A reduction of the basal insulin dose after exercise based on its intensity and duration is also recommended. After physical activity, the blood glucose level should be checked and a supplement of approximately 15–20g of CHs should be taken if the value is less than 120mg/dL. The time of CH intake after exercise affects glycogen synthesis in the long term: intake within 30min of exercise (1.0–1.5g CH/kg at 2h intervals up to 6h) induces higher glycogen levels as compared to when intake is delayed for 2h.51
In patients with T2DM treated with insulin and/or SUs or repaglinide there is also an increased risk during and after exercise, especially if the prior blood glucose level is less than 100g/dL.44 To prevent hypoglycemia, it is advisable to decrease oral medication52 or insulin dose before and possibly after exercise.52,53 For long-lasting (more than 60–90min) or unplanned exercise, the intake of CH supplements should be based on exercise duration and intensity.43 Once the activity is completed, it is advisable to verify blood glucose levels and to take 15–20g of CHs if the value is lower than 120mg/dL.
Nutritional management of hypoglycemiaRecommendations- -
We recommend the measurement of CH content by counting, exchange, or estimation based on experience as an essential strategy to achieve good glucose control and to prevent hypoglycemia in patients on insulin therapy (1⊕⊕⊕○).
- -
We recommend a diet with a low glycemic index to decrease episodes of hypoglycemia in both children and adults (1⊕⊕○○).
- -
During an acute intercurrent condition, in addition to adequate hydration and CBGSM, we recommend adequate CH intake to prevent hypoglycemia (1⊕⊕⊕○).
Although the optimum composition in macronutrients of the diet for patients with DM is controversial,54 it is universally accepted that the management of CH contents is essential for adequate glycemic control.55 The American Diabetes Association recommends CH counting as the best way to control blood glucose, simultaneously promoting whole grain and fiber consumption.56,57
In addition to CH content, the glycemic index (GI) and glycemic load are also important. Various clinical studies suggest that diets with a low GI are particularly effective in the event of insulin resistance, overweight or obesity and insulin treatment.58 A Cochrane meta-analysis has shown that a diet with CHs of low GI improves glycemic control, reduces cardiovascular risk, and decreases the risk of hypoglycemia.59
CH counting, type, and distribution are particularly important in patients on rapid insulin.60,61 If intensive insulin therapy is administered, education by experienced professionals is essential to maintain safety in making estimates.62 Treatment with premixed insulin requires the administration of food whose CH content is estimated on a regular basis in accordance with requirements, while in basal-bolus or CSII therapy, the estimation of the CH content in intake allows the rapid insulin dose needed to be adapted as necessary.
Moderate amounts of alcohol taken with food do not significantly increase the risk of hypoglycemia, but high alcohol intake or isolated alcohol intake without CH increase the risk.63
Drug interventions in diabetes mellitus: Oral therapyRecommendations- -
We recommend the use of metformin as the first option in T2DM because of the low risk of hyperglycemia and its beneficial effects on metabolic parameters and, possibly, on cardiovascular morbidity and mortality (1⊕⊕⊕⊕). If metformin is contraindicated or not tolerated, monotherapy with an oral drug with a low risk of hypoglycemia is recommended, particularly in patients with some risk factor for severe hypoglycemia (1⊕⊕⊕○).
- -
We recommend that, before treatment is started with SUs or repaglinide, the risk factors for hypoglycemia and any potential interactions with drugs that may their enhance their hypoglycemic action should be assessed (1⊕⊕⊕⊕).
- -
We recommend the avoidance of long-acting SUs such as chlorpropamide or glibenclamide because of their greater risk of hypoglycemia (1⊕⊕⊕⊕).
- -
We recommend the immediate re-evaluation of the treatment scheme in patients with severe or unaware hypoglycemia treated with SUs or repaglinide, and that the use of another drug with no risk of hypoglycemia be considered (1⊕⊕⊕○).
- -
We suggest that, in T2DM patients with dual therapy, HbA1c near the treatment goal, and a high risk of hypoglycemia, a triple combination of drugs not inducing hypoglycemia should be used (2⊕⊕○○).
A recent review by the Agency for Healthcare Research and Quality64 showed a mild to moderate risk of hypoglycemia three to sevenfold higher for SUs or glinides, with no difference between them as compared to metformin, glitazones, or DPP4 inhibitors (DPP4I), also with no differences between them. Regarding the incidence of severe hypoglycemia, there were no differences between the different monotherapies. The review concluded by recommending metformin as the drug of first choice as monotherapy because of its effects on HbA1c, weight, and lipids, together with its low risk of hypoglycemia and low costs. Two drug classes not included in this review, alpha-glucosidase inhibitors and sodium-glucose cotransporter-2 (SGLT2) inhibitors, show an incidence of hypoglycemia similar to placebo as monotherapy. Alpha-glucosidase inhibitors have shown an incidence of hypoglycemia similar to metformin or DPP4Is but lower than SUs.65–68
The prevalence of mild hypoglycemia in patients taking SUs ranges from 16% to 20%.69 We recommend the assessment of the risk factors for hypoglycemia (Table 1) and potential interactions with drugs that may enhance the hypoglycemic action of SUs or glinides (Table 2) before treatment is started with these drugs,8,70 with another therapeutic class being selected if the patient is at risk of hypoglycemia. The use of longer acting SUs (chlorpropamide, glibenclamide) should be avoided because they induce hypoglycemia more commonly than short-acting SUs (glipizide, gliclazide, glimepiride) or glinides.71–73
Risk factors for hypoglycemia in type 2 diabetes mellitus.
| Conventional risk factors (absolute or relative insulin excess) |
| Excess, inadequate, or erroneous dose of insulin or secretagogue |
| Use of long-acting sulphonylureas (glibenclamide, chlorpropamide) |
| Decreased exogenous glucose provision (nighttime fast, missed meals, malnutrition) |
| Increased glucose utilization (exercise) |
| Decreased endogenous glucose production (alcohol intake, liver insufficiency) |
| Increased insulin sensitivity (after weight loss, increase in regular physical exercise, improved glycemic control, or during the night) |
| Decreased insulin clearance (renal failure) |
| Risk factors for autonomic insufficiency associated with hypoglycemia |
| Absolute endogenous insulin deficiency |
| History of severe, unaware, or recent hypoglycemic episodes |
| Aggressive blood glucose control |
| Risk factors for severe consequences after hypoglycemia |
| Hazardous occupations (drivers, work at heights, security forces, etc.) |
| Heart disease: coronary artery disease, arrhythmia |
| Elderly patients |
| Patients with frequent hospital admissions |
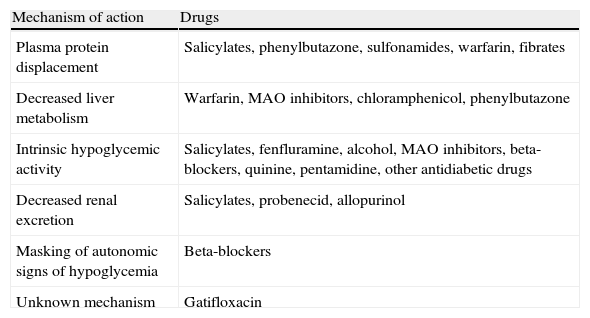
Interactions with drugs that may enhance hypoglycemic action.
| Mechanism of action | Drugs |
| Plasma protein displacement | Salicylates, phenylbutazone, sulfonamides, warfarin, fibrates |
| Decreased liver metabolism | Warfarin, MAO inhibitors, chloramphenicol, phenylbutazone |
| Intrinsic hypoglycemic activity | Salicylates, fenfluramine, alcohol, MAO inhibitors, beta-blockers, quinine, pentamidine, other antidiabetic drugs |
| Decreased renal excretion | Salicylates, probenecid, allopurinol |
| Masking of autonomic signs of hypoglycemia | Beta-blockers |
| Unknown mechanism | Gatifloxacin |
MAO: monoamine oxidase.
As a second option, the review by the Agency for Healthcare Research and Quality showed an increased risk of mild or moderate hypoglycemia in patients with combination therapies as compared to monotherapy.64 The relative risk (RR) of hypoglycemia was 5.8 for the SU-metformin versus the glitazone-metformin combination, and 1.8 for the metformin-glinide versus the metformin-glitazone combination, with no significant differences between the metformin-glitazone and metformin-DPP4I combinations.
In two meta-analyses, SUs and glinides were associated with 4.6–8.9-fold and 7.5–10.5-fold increases respectively in the risk of hypoglycemia as compared to placebo. No increased risk was seen with glitazones, alpha-glucosidase inhibitors, or DPP4Is compared to placebo.74,75 The incidence of hypoglycemia with SGLT2 inhibitors in dual or triple therapy was similar to placebo, and lower than glipizide in the case of dapagliflozine (3.4% vs. 39.7%).76
Some consensuses consider the possibility of adding a third non-insulin drug with an action mechanism complementary to that of dual therapy when metabolic control of the patient is poor and HbA1c is not very high (less than 8.5%).77 If the risk of hypoglycemia is high, drugs with a low risk of hypoglycemia should be selected.
Drug interventions in diabetes mellitus: Subcutaneous therapiesRecommendations- -
We recommend the use of basal insulin analogs to decrease the risk of hypoglycemia, especially nocturnal hypoglycemia (1⊕⊕⊕○).
- -
We recommend the use of basal insulin analogs to decrease the risk of postprandial hypoglycemia (1⊕⊕○○).
- -
In patients treated with CSII, we recommend the use of rapid insulin analogs (aspart or lispro) to decrease the risk of hypoglycemia (1⊕⊕○○).
- -
In patients with DM and obesity, we recommend the use of glucagon-like peptide (GLP-1) agonists as second or third-line treatment because of their low risk of hypoglycemia, antidiabetic potency, and additional weight reduction effects (1⊕⊕⊕○).
The use of insulin analogs has been shown to decrease the risk of hypoglycemia as compared to human insulins. As regards basal insulin analogs, in patients with T1DM both glargine78 and detemir79 have been shown to decrease the incidence of total hypoglycemia, particularly nocturnal hypoglycemia. In patients with T2DM, basal analogs are associated with a lower risk of hypoglycemia, especially at night (50% reduction), as compared to insulin NPH.80,81 In a meta-analysis of the National Institute for Health and Clinical Excellence, hypoglycemia rates were significantly lower in patients treated with insulin glargine (RR: 0.89; 95% CI: 0.83–0.96) or insulin detemir (RR: 0.68; 95% CI: 0.54–0.86) as compared to NPH.82
Rapid insulin analogs have also been shown to decrease the frequency of hypoglycemia as compared to regular insulin.83–86 Finally, in patients on CSII, both aspart and lispro decrease the hypoglycemia rate and induce a better control of postprandial blood glucose.87–90
Degludec is a new basal insulin analog with a longer duration of action which achieves a glycemic control similar to glargine, but with a greater effect on basal blood glucose. In a recent meta-analysis,91 the incidence of hypoglycemia was lower in patients treated with degludec as compared to glargine (T2DM, RR of total hypoglycemia: 0.83, and 95% CI: 0.74–0.94; RR of nocturnal hypoglycemia: 0.68, and 95% CI: 0.57–0.82; T1DM, RR of nocturnal hypoglycemia: 0.75, and 95% CI: 0.60–0.94).
GLP-1 receptor agonists constitute a therapeutic class which provides in patients with T2DM a potent normoglycemic effect together with a low risk of hypoglycemia and positive effects on weight.92 Hypoglycemic episodes mainly occur in association with SUs or insulin.92 In the clinical trial program Liraglutide Effect and Action in Diabetes (LEAD), the overall incidence of hypoglycemia associated with liraglutide ranged from 0.03 to 1.9 events/patient/year, with no difference from placebo for the 1.2mg dose.93 Treatment with liraglutide 1.8mg also involved a low risk of hypoglycemia, although slightly higher than placebo, in studies in combination with SUs: 0.47 vs 0.17 events/patient/year in the LEAD-1-SU study94; 0.6 vs 0.2 events/patient/year in LEAD-495; 0.06 and 1.2 (greater and lower hypoglycemia rate respectively) vs 0 and 1.0 events/patient/year in LEAD-5.96
Treatment with exenatide is also associated with a low risk of hypoglycemia. In the studies Diabetes Therapy Utilization: Researching Changes in A1C, Weight and Other Factors Through Intervention with Exenatide Once Weekly [DURATION] 1–5, 13% of patients treated with exenatide weekly and 16% of those treated with exenatide twice daily experienced some episode of minor hypoglycemia, but the incidence in patients not treated with SUs was 1% and less than 1%, respectively.97 In head-to-head clinical trials, the incidence of minor hypoglycemia was lower in the liraglutide versus the exenatide arm (liraglutide: 1.93 vs exenatide: 2.60 events/patient/year; RR: 0.55; 95% CI: 0.34–0.88; p=0.0131) and similar to exenatide weekly (liraglutide: 10.8% vs exenatide weekly: 8.9%; p=0.374), despite a significantly higher effect of liraglutide on glycemic control.93,97,98 In patients inadequately controlled on basal insulin with or without SU, the incidence of symptomatic hypoglycemia was more frequent with a new analog, lixisenatide (42.9%), compared to placebo (23.6%), but similar in both groups (32.6% vs 28.3%) when patients treated with SU were excluded, and there were no cases of severe hypoglycemia.99
Other conditions influencing hypoglycemiaRecommendations- -
We recommend that increased attention be paid to diabetic patients with a low body mass index (BMI) and long disease duration because of the increased risk of severe hypoglycemia (1⊕⊕⊕○).
- -
We recommend an intensification of care to prevent hypoglycemia in patients with renal failure, autonomic neuropathy (1⊕⊕⊕⊕), or the presence of peripheral ulcers (1⊕⊕○○).
BMI is inversely associated with the risk of severe hypoglycemia. A BMI greater than 30kg/m2 was associated with a 35% lower incidence of severe hypoglycemia than a BMI lower than 25kg/m2 (HR: 0.65; 95% CI: 0.5–0.85).100 In the ADVANCE study,4 the risk of severe hypoglycemia decreased 5% per each unit increase in the BMI 0.95; 95% CI: 0.93–0.98). The duration of DM also represents a risk factor for severe hypoglycemia in both T2DM101 and T1DM patients,102 with a 2% per year risk increase after 10–15 years.4
Renal failure100 and peripheral neuropathy103 are also associated with a greater risk of severe hypoglycemia, while the presence of peripheral ulcers is positively associated with a risk of hospitalization for hypoglycemia (OR: 1.71; 95% CI: 1.2–2.44).104
Hypoglycemia in special situationsPregnancyRecommendations- -
We recommend strict glycemic control before pregnancy and in the first trimester, to avoid both blood glucose fluctuations and hypoglycemia (1⊕⊕○○).
- -
We recommend diabetic education of the patients and of the people around them to ensure the effective prevention and treatment of hypoglycemia. CBGSM is advised before and one hour after meals, at bedtime, and between 2:00 and 4:00 AM if nocturnal hypoglycemia is suspected (1⊕⊕○○).
- -
We recommend the use of detemir as basal insulin, along with rapid insulin analogs (aspart and lispro) (1⊕⊕○○).
Glycemic control goals during pregnancy are more stringent than normal,2 so increasing the risk of hypoglycemia,105 particularly unaware and severe hypoglycemia, during the first trimester of pregnancy. Impaired counterregulatory response to hypoglycemia and the presence of nausea and vomiting contribute to this situation.
Risk factors for severe hypoglycemia include a history of severe hypoglycemia in the previous year, unaware hypoglycemia, the length of DM duration, low pregestational HbA1c, fluctuating blood glucose, and excess use of supplemental insulin injections. Its distribution is not homogeneous, so that 10% of patients experience 60% of events.106
The rapid-acting analogs lispro and aspart are safe in pregnancy107 and induced a lower frequency of hypoglycemia and glucose fluctuations in some studies, but not all.86 The use of CSII has no clear benefits in pregnancy with regard to the risk of hypoglycemia.108 As regards basal analogs, glargine appears to be safe,109 but no adequate studies supporting its use in pregnancy are available yet, while the use of detemir110 and NPH is approved (category B for both).
Elderly patientsRecommendations- -
We recommend individualized control goals in the elderly, with the risk of hypoglycemia being a priority consideration (1⊕⊕⊕○).
- -
We recommend that diabetic education be adapted to the patients and to those living with them, to ensure the effective prevention and treatment of hypoglycemia (1⊕⊕○○).
- -
We suggest CBGSM, especially if there is any change in neurological status (2⊕○○○).
Hypoglycemia is common in elderly patients with DM, who are more prone to experience hypoglycemia because of their poor physical, nutritional and cognitive state, counterregulatory response, and reaction capacity.111 Comorbidities and polypharmacy increase the risk of severe and unaware hypoglycemia even further.112 These patients mainly have neuroglycopenic clinical symptoms, and are at risk of neurological113,114 and physical damage (falls, arrhythmia, etc.).115
Severe hypoglycemia has a great impact on treatment adherence, quality of life,116 and even mortality in the elderly,101,117 causing frequent hospital admission118 usually related to treatment with SUs or insulin. Intensive treatment to achieve strict glycemic control is associated with an increased risk of severe hypoglycemia, and individualized treatment taking into account age,101 impaired renal function, slowing in hormonal regulation and counterregulation, hydration status, variable appetite, nutritional intake, and polypharmacy as risk factors is therefore required.119
Treatment of hypoglycemiaRecommendations- -
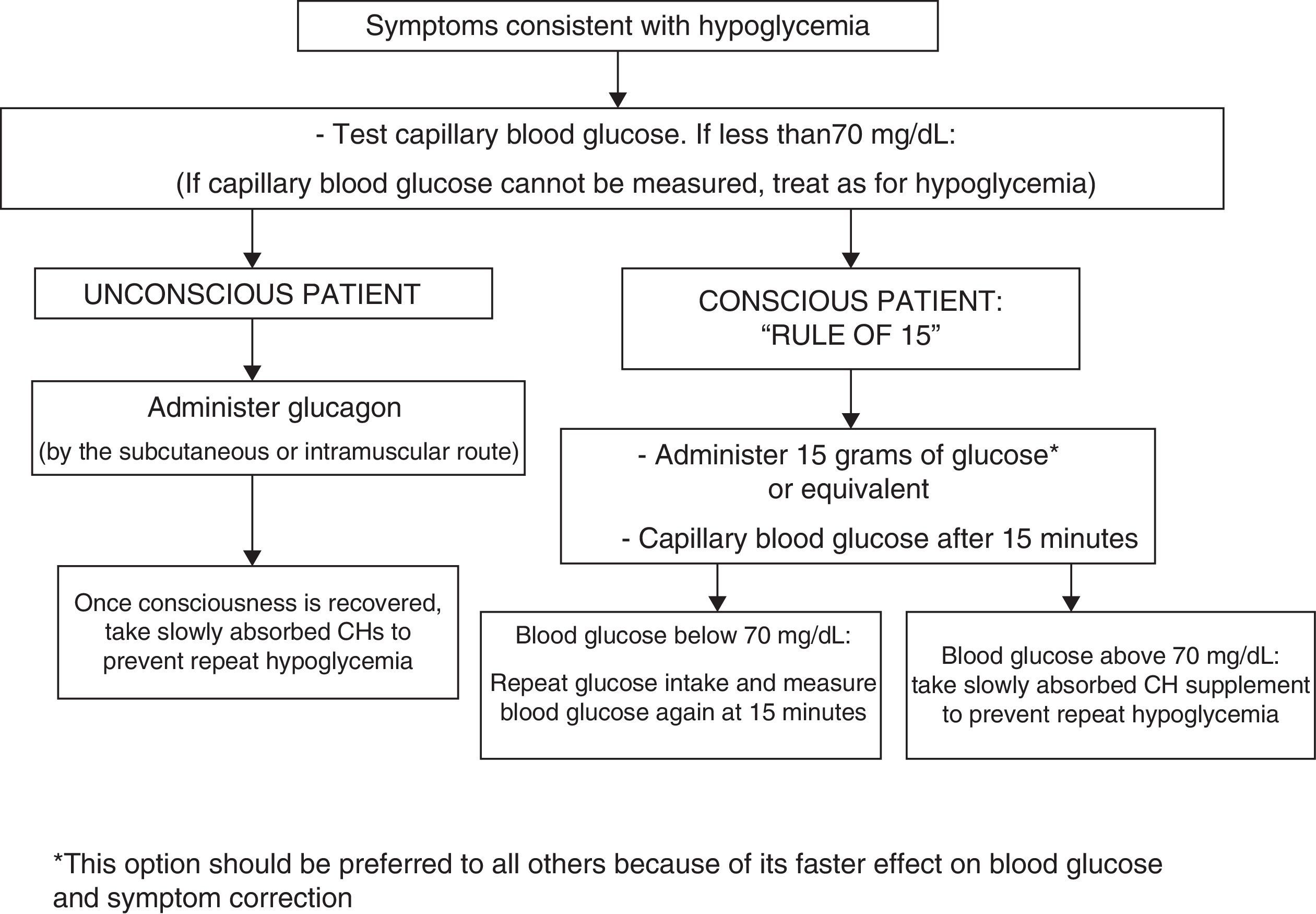
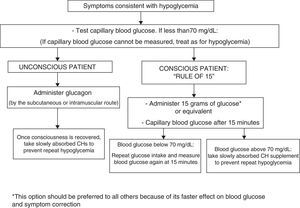
In conscious patients, we recommend that episodes of hypoglycemia should preferably be treated with 15g of glucose, or with any CH containing this amount. This treatment should be repeated at 15min if a capillary blood glucose measurement shows persistent hypoglycemia. Once normal blood glucose levels have been restored, we recommend the intake of a slowly absorbed CH supplement to prevent hypoglycemia (1⊕⊕○○).
- -
In unconscious patients, we recommend the administration of glucagon by subcutaneous injection (1⊕⊕○○).
- -
In patients treated with insulin, SUs, or repaglinide, we recommend the regular evaluation of knowledge concerning the detection and treatment of hypoglycemia, and that the need to always have available adequate CHs to treat hypoglycemia and glucagon should be kept in mind (1⊕○○○).
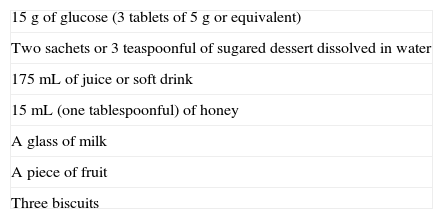
To treat hypoglycemia (a capillary blood glucose level less than 70mg/dL), the administration of 15g glucose (Fig. 1) or its equivalent is recommended2,120 (Table 3). In a randomized study of 41 patients with T1DM which compared the effect of seven treatment methods (glucose as solution, tablets or gel, sucrose solution or tablets, hydrolyzed polysaccharide solution, and orange juice),121 a similar increase in blood glucose levels was seen with all agents, except for gel and orange juice, which had no effect at 10min and caused a lower glucose elevation at 20min.121 Treatment with oral glucose is therefore preferred because of its faster effect on blood glucose and symptom improvement121 as compared to other options (milk or orange juice) which have a slower effect.122 However, if glucose is not available, the intake of any CH is valid.
The treatment of hypoglycemia with fat-rich food (sweets, chocolate) is not recommended because this delays CH absorption and may result in a greater subsequent hyperglycemic excursion. The intake of preparations containing caffeine and/or fructose in addition to glucose is not recommended either because of a lack of evidence about their effects. If the patient has symptoms consistent with hypoglycemia but no glucometer is available for confirmation, it is recommended that the condition be managed as hypoglycemia.120
Severe episodes of hypoglycemia may require the administration of glucagon by subcutaneous or intramuscular injection, and checks should be regularly carried out to ensure that patients have glucagon available. Glucagon effects are impaired in patients with advanced liver disease and in those who have drunk alcohol (more than two units) in the previous hours.120 No differences have been seen as regards the glucagon administration route (subcutaneous versus intramuscular).123 For severe hypoglycemia in a healthcare setting, and provided venous access is available, the administration of 50% glucose (50mL IV) is preferred over glucagon (IV or IM) because of its faster effect in the restoration of blood glucose.124,125
It is also important that all patients receiving treatment with insulin and/or SUs or repaglinide be trained not only in the recognition of symptoms of hypoglycemia, but also in measures for the adequate treatment of the condition. An understanding of such measures should regularly be re-evaluated during patient monitoring. Patients should also be reminded of the need to take with them sufficient CHs to treat an episode of hypoglycemia.
Unaware hypoglycemiaRecommendations- -
We recommend that both conventional risk factors and risk factors suggesting impaired counterregulation be taken into consideration with regard to patients with repeated hypoglycemic episodes (1⊕⊕⊕⊕).
- -
In patients with asymptomatic hypoglycemia, we recommend the prevention of hypoglycemia for at least two to three weeks to improve the perception of hypoglycemia (1⊕⊕○○).
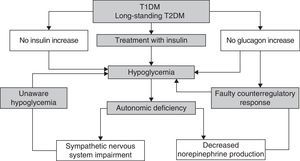
Recurrent hypoglycemic episodes decrease sympathoadrenal and glucagon counterregulatory response in a vicious cycle which causes the patent to be more prone to episodes of unaware hypoglycemia1 (Fig. 2).
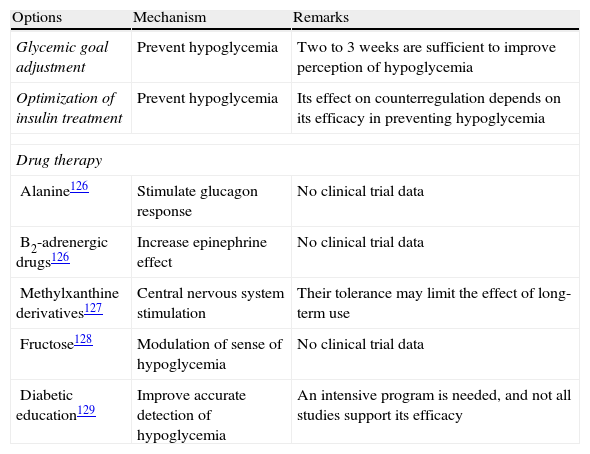
There are different strategies for the prevention and management of unaware hypoglycemia (Table 4).126–129 The main measure is the avoidance of hypoglycemia so as to reverse the loss of a counterregulatory response, which may improve the perception of hypoglycemia after approximately three days and so restore the response to hypoglycemia in about three weeks.
Strategies for the prevention and management of unaware hypoglycemia.
| Options | Mechanism | Remarks |
| Glycemic goal adjustment | Prevent hypoglycemia | Two to 3 weeks are sufficient to improve perception of hypoglycemia |
| Optimization of insulin treatment | Prevent hypoglycemia | Its effect on counterregulation depends on its efficacy in preventing hypoglycemia |
| Drug therapy | ||
| Alanine126 | Stimulate glucagon response | No clinical trial data |
| B2-adrenergic drugs126 | Increase epinephrine effect | No clinical trial data |
| Methylxanthine derivatives127 | Central nervous system stimulation | Their tolerance may limit the effect of long-term use |
| Fructose128 | Modulation of sense of hypoglycemia | No clinical trial data |
| Diabetic education129 | Improve accurate detection of hypoglycemia | An intensive program is needed, and not all studies support its efficacy |
In patients with T1DM and unaware hypoglycemia, CSII reduces episodes of hypoglycemia by half, and is especially effective in decreasing severe hypoglycemic episodes (from 1.25 to 0.05 events/year).130 If severe recurrent hypoglycemic episodes occur, pancreas and pancreatic cell islet transplant should be considered as a treatment option.10
Metabolic control goal in patients with hypoglycemiaRecommendations
- -
We recommend that less aggressive glycemic control goals be established in patients with DM who have experienced hypoglycemia (particularly if severe) or when their risk of hypoglycemia is considered to be high (1⊕⊕⊕⊕).
- -
We recommend for these patients an HbA1c goal ranging from 7% to 8%, or a higher goal if a very high risk exists (2⊕⊕○○).
- -
We recommend flexibility in setting glycemic control goals in patients at a high cardiovascular risk (1⊕⊕⊕○).
- -
We recommend that more ambitious control goals be established in patients with T2DM if antidiabetic treatment includes drugs with a low risk of hypoglycemia (1⊕⊕⊕○).
- -
We suggest that glycemic variability be reduced to decrease the risk of hypoglycemia and to achieve more stringent HbA1c goals (2⊕⊕○○).
Intensive DM treatment is associated with a greater risk of hypoglycemia. In T2DM, two recent meta-analysis showed a greater risk of severe hypoglycemia in the intensive treatment group (HR: 2.48; 95% CI: 1.91–3.21)131 and (OR: 3.01, CI: 1.47–4.60).132 Some patient characteristics are associated with a greater risk of hypoglycemia and these should be taken into consideration in order to establish a less strict glycemic control goal (Table 1).
Among insulin-treated patients, the risk of hypoglycemia is higher in those with lower mean blood glucose and greater glycemic variability (measured as standard deviation in CBGSM).133 It is therefore concluded that the risk of hypoglycemia associated with treatment intensification in patients with T2DM could be minimized by addressing this reduction in glycemic variability. In this regard, it has been suggested that glycemic variability may influence the risk of hypoglycemia irrespective of the type of treatment used (insulin sensitizers, oral secretagogue drugs, or insulin) and overall glycemic control.134
Although less stringent glycemic control goals are recommended for patients at risk of severe hypoglycemia,135 no specific HbA1c and, especially, basal and postprandial glucose values are given, and the clinical criterion is therefore irreplaceable.
Value of capillary blood glucose self-monitoringRecommendations- -
We recommend CBGSM when hypoglycemia is suspected, after treatment for hypoglycemia until normal blood glucose levels are restored, and before activities that may increase the risk of hypoglycemia (exercise) or are potentially dangerous (driving, child care, hazardous work) are performed (1⊕⊕⊕⊕).
- -
We recommend regular verification of the CBGSM procedure and its results, as well as of the extent to which it is possible to make adequate decisions based on them (1⊕○○○).
- -
We recommend continuous glucose monitoring (CGM) in patients with unaware or frequent hypoglycemia (1⊕○○○).
- -
We suggest the use of CGM in patients with T1DM because this decreases the time spent in hypoglycemia as compared to CBGSM, but not the number of severe or total hypoglycemic episodes (1⊕⊕⊕○).
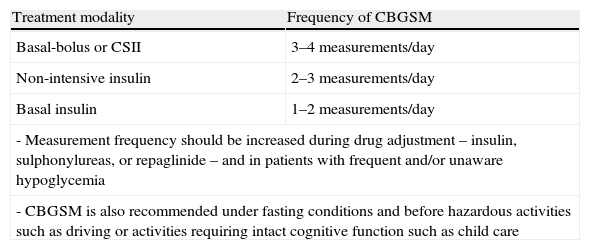
CBGSM is clearly important as an integral part of the intensive treatment of T1DM and T2DM patients treated with SUs or repaglinide and/or insulin, because it minimizes the risk of hypoglycemia and helps to identify it. The recommended frequency of CBGSM is summarized in Table 5.2,136
Recommendations for capillary blood glucose self-monitoring.
| Treatment modality | Frequency of CBGSM |
| Basal-bolus or CSII | 3–4 measurements/day |
| Non-intensive insulin | 2–3 measurements/day |
| Basal insulin | 1–2 measurements/day |
| - Measurement frequency should be increased during drug adjustment – insulin, sulphonylureas, or repaglinide – and in patients with frequent and/or unaware hypoglycemia | |
| - CBGSM is also recommended under fasting conditions and before hazardous activities such as driving or activities requiring intact cognitive function such as child care | |
In children and adolescents with T1DM, an increased frequency of CBGSM was associated with HbA1c improvement (by up to 20% per additional measurement between 2 and 5 measurements daily) and less acute complications, including hypoglycemia.137 The benefit to quality of life or to metabolic control in patients with T2DM not treated with insulin remains controversial, and the data on its efficacy for the prevention of hypoglycemia are conflicting.138,139
The accuracy of CBGSM is highly dependent on the user and the instrument.140 The procedure to be used, the need for CBGSM, its frequency and time of performance, as well as patient understanding of the measures to be taken based on CBGSM should be reviewed regularly.140
The effect of CGM on severe hypoglycemia has not been fully established.141,142 It could be useful in patients with very frequent and/or unaware hypoglycemia, especially those with good control. In patients with T1DM with HbA1c less than 7.5%, CGM decreased time in hypoglycemia (0.48h/day vs 0.97h/day; p=0.03), but not the number of hypoglycemic episodes, improving glycemic control and with no severe hypoglycemia.143 However, it has not shown clear benefits regarding the risk of hypoglycemia (severe and non-severe) as compared to CBGSM,144–148 except for a slight reduction in time in hypoglycemia (23% or 15min/day).148–150
Hypoglycemia and occupational activityRecommendations- -
We suggest that the employer assigns regular shifts to patients with DM and allows for capillary blood glucose self-monitoring and CH intake during working hours (2⊕○○○).
- -
We suggest that current regulations for obtaining licenses for hazardous activities (vehicle driving, guns, air security, state security forces) be adapted to new realities, such as the use of drugs with less risk of hypoglycemia (2⊕○○○).
The occurrence of hypoglycemia is a limiting factor for workers with DM, and even an exclusion factor in certain occupations. This increases work absenteeism and decreases productivity and quality of life.6 The individualized study of each patient by the physician, the selection of therapies with a low risk of hypoglycemia, and patient education in the management of hypoglycemia are essential for maintaining occupational quality and safety standards.151
The advent of new drugs with a low risk of hypoglycemia has allowed for the access of patients with T2DM to activities where hypoglycemia has traditionally been considered a problem: public transport, planes, trains, supervision of air or land traffic, occupations related to guns (police, army) or risk of fall (electrician, work on roofs). In some of these, DM treated with insulin continues to be a reason for exclusion. In all other occupations, limitations are based on the frequency or severity of hypoglycemic episodes.152
According to current Spanish air safety legislation,152 subjects with T1DM can neither obtain nor continue to hold a flying license. In subjects with TD2M, only metformin and alpha-glucosidase are allowed; there are no updated recommendations regarding incretin and thiazolidinedione therapy.
To obtain a group 1 driving license (A1, A2, B1, and B2), patients should not have DM with severe metabolic instability or requiring hospital care, while for group 2 licenses (C1, C2, D, and E) T1DM patients are also excluded.153 A favorable medical report is mandatory for license renewal, and report validity is shorter for patients with T1DM or T2DM on insulin as compared to all other patients. The applicable law for state security forces excludes subjects with DM irrespective of treatment and the risk of hypoglycemia.
Variable work shifts are advised against “in insulin-dependent diabetics, although they may be adapted, with education and by tailoring diet and insulin, to work requirements”. Night shift work is feasible if the patient is able to adapt his/her insulin requirements. In healthcare staff (physicians or nurses), exclusion from these shifts is less justified “because of their knowledge and access to healthcare resources in the event of an emergency”. It is also recommended that extreme temperatures that may cause dehydration or trigger hypoglycemia be avoided.154
FundingThis consensus document has received external funding consisting of a scholarship from the Fundación de la Sociedad Española de Endocrinología (FSEEN), through an unrestricted grant of the pharmaceutical companies Novo Nordisk and FAES Farma. The sponsors have exerted no influence on any phase of the preparation of the document and have had no access to its contents.
Contribution to preparation of the manuscript – author (subject)Pharmaceutical companies: A: Almirall; AZ: Astra-Zeneca; B: Boehringer Ingelheim; BMS: Bristol-Myers Squibb; E: Esteve; F: Ferrer; FF: Faes Farma; GSK: Glaxo Smith Kline; I: Intarctia J: Janssen-Cilag; L: Lilly; MSD: MSD; N: Novartis; NN: Novo Nordisk; R: Roche; SA: Sanofi-Aventis; T: Takeda.
Ó Moreno (Definition and classification of hypoglycemia), speaker/consultant: NN, L, MSD, N, B. JF Merino (Hypoglycemia in type 1 diabetes), clinical investigator: NN, L, SA, MSD, AZ; speaker/consultant: NN, L, MSD, N, B, AZ, BMS, E, SA, FF. M Botella (Hypoglycemia in type 2 diabetes), clinical investigator: SA, L, MSD; speaker: NN, L, SA, MSD, R, GSK. M Gargallo (Hypoglycemia and cardiovascular disease), speaker/consultant: AZ, BMS, B, NN, MSD, SA. M Muñoz (Hypoglycemia and risk of fracture in patients with diabetes mellitus), clinical investigator: NN, J, GSK; speaker/consultant: NN, GSK, FF. J Escalada (Hypoglycemia and physical exercise), speaker/consultant: AZ, B, BMS, L, NN, MSD, SA, A. D Bellido (Nutritional management of hypoglycemia), clinical investigator: L, R. SA, MSD, NN; speaker/consultant: L, N, NN, AZ, E, SA. JJ Gorgojo (Drug interventions in diabetes mellitus: oral therapy), consultant/speaker/investigator: NN, L, SA, GSK, A, N, MSD, BMS, AZ, D, F. R Reyes (Drug interventions in diabetes mellitus: subcutaneous therapies), clinical investigator: NN, J; speaker/consultant: NN, SA, GSK, FF, A, N. A. Becerra (Other conditions influencing hypoglycemia), clinical investigator: NN, L, GSK, N, R, SA; speaker/consultant: L, NN, GSK, N, R, SA, A, AZ, E, FF. M López de la Torre (Hypoglycemia in special situations), consultant/speaker/investigator: NN, L, SA, GSK, A, N, MSD, BMS, FF, AZ, D, F. P Mezquita (Treatment of hypoglycemia), clinical investigator: L, R. SA, NN, MSD, B; speaker/consultant: L, A, N, NN, AZ, BMS, E, MSD, SA, FF. A Soto (Unaware hypoglycemia), clinical investigator L, R, SA, NN, T; speaker/consultant: L, A, T, N, NN, AZ, E, SA, FF. F Gómez Peralta (Metabolic control goal in patients with hypoglycemia), investigator: L, SA, NN, B; speaker/consultant: L, N, NN, AZ, BMS, E, MSD, as. E Jódar (Value of capillary blood glucose self-monitoring), investigator B, GSK, J, L, MSD, NN, I; speaker/consultant: FF, L, N, NN; N González (Hypoglycemia and occupational activity), speaker NN, L, B, L, R, N, MSD, GSK, FF.
Conflicts of interestNo author has reported any relevant conflict of interest with regard to the preparation of this article. The final version of the article has been approved by all the authors. All the authors have contributed equally to the preparation of the document.
Please cite this article as: Mezquita-Raya P, Reyes-García R, Moreno-Pérez Ó, Muñoz-Torres M, Merino-Torres JF, Gorgojo-Martínez JJ, et al. Documento de posicionamiento: evaluación y manejo de la hipoglucemia en el paciente con diabetes mellitus. Grupo de Trabajo de Diabetes Mellitus de la Sociedad Española de Endocrinología y Nutrición. Endocrinol Nutr. 2013;60:517.