There is no consensus on the remission criteria for Cushing's disease or on the definition of disease recurrence after transsphenoidal surgery, and comparison of the different published series is therefore difficult. A long-term recurrence rate of Cushing's disease ranging from 2% to 25% has been reported. Predictors of long-term remission reported include: (1) adenoma-related factors (aggressiveness, size, preoperative identification in MRI), (2) surgery-related factors, mainly neurosurgeon experience, (3) clinical factors, of which dependence on and duration of glucocorticoid treatment are most important, and (4) biochemical factors. Among the latter, low postoperative cortisol levels, less than 2mcg/dL predict for disease remission. However, even when undetectable plasma cortisol levels are present, long-term recurrence may still occur and lifetime follow-up is required. We report the preliminary results of the first 20 patients with Cushing's disease operated on at our hospital using nadir cortisol levels less than 2mcg/dL as remission criterion.
En el manejo de la enfermedad de Cushing (EC) no existe un consenso sobre los criterios de remisión ni sobre la definición de recurrencia en la literatura, por lo que las series no son comparables. Se ha descrito que la tasa de recurrencia en la EC oscila entre 5-25% en el seguimiento a largo plazo. Dentro de los factores pronósticos de remisión a largo plazo podemos diferenciar: 1) factores dependientes del adenoma (agresividad, tamaño, identificación preoperatoria por técnicas de imagen); 2) factores dependientes de la cirugía, donde destaca por su importancia la experiencia del neurocirujano; 3) factores clínicos, siendo la dependencia del tratamiento glucocorticoideo y su duración los más demostrados; y 4) factores bioquímicos. Dentro de estos últimos queda bien documentado en la literatura que un nadir indetectable de cortisol, al menos inferior a 2mcg/dL, en el postoperatorio predice la remisión de la enfermedad pero, incluso en estos casos, no puede excluirse la recidiva, lo que obliga al seguimiento de por vida en estos pacientes. Presentamos los resultados preliminares de los primeros 20 pacientes intervenidos en el Hospital Universitario de la Ribera utilizando el nadir de cortisol inferior a 2mcg/dL.
Cushing's disease (CD) is a rare condition with an incidence rate of 0.7–2.4 cases per million inhabitants, per year.1–3 CD is characterized by hypercortisolism caused by a pituitary adenoma secreting adrenocorticotropic hormone (ACTH). ACTH-secreting pituitary adenomas are the most common cause of endogenous hypercortisolism, accounting for 65–70% of cases of endogenous Cushing's syndrome. CD causes obesity, diabetes mellitus, high blood pressure, muscle weakness, osteoporosis, depression, and cognitive disturbances, and a 5-year cardiovascular mortality risk of up to 50% in untreated patients. Inadequately treated CD patients have a standardized mortality rate five times higher than normal, which decreases to normal in patients who achieve normal cortisol levels after surgery.2,3
Although the clinical, biochemical, and imaging characteristics of CD have been well known for decades, both the diagnosis and long-term management of CD continue to represent a challenge.
The treatment of choice of CD is transsphenoidal surgery with resection of the pituitary adenoma, which is a microadenoma in 95% of cases.4 Ideally, complete and selective resection of corticotropic microadenomas should cure CD without adversely affecting the remaining pituitary function. With advances in transsphenoidal microsurgery, remission rates in the early postoperative period range from 55% to 85%5,6 depending on the different series. Despite therapeutic advances, CD has a long-term recurrence rate of 10–15%, which reaches almost 25% in studies with longer follow-up times (20 years after surgery), and lifetime follow-up should therefore be performed.7–9
One of the most controversial issues in CD management is the establishment of “cure” or “remission” criteria, although the definition of remission is preferred because of the possibility of recurrence (as cure would involve final resolution). An ideal definition of “remission” of CD should be available in the early postoperative period and should be associated with the reversion of the clinical characteristics and the normalization of the biochemical parameters of CD.10 The most recent guidelines on CD management, published in 2008, redefine remission as occurring when cortisol levels ranging from 2 to 5mcg/mL are achieved, while the persistence of high or only moderately decreased cortisol or urinary free cortisol levels suggests recurrence. Disease remission should be assessed from both the clinical and biochemical viewpoints.5
Clinical remission and persistence of diseaseAfter transsphenoidal surgery, some patients experience a dramatic and rapid resolution of the clinical signs of CD. However, resolution takes longer to occur in most cases. Despite biochemical normalization after adequate treatment, hypercortisolism may have a negative effect in the long term: the persistence of increased cardiovascular risk for at least five years after surgery, the lack of a restoration of the nocturnal dipper pattern, persistent high blood pressure in children with CD, impaired glucose tolerance, and cognitive impairment.11–15 Recovery as measured in Health-Related Quality of Life (HRQol) questionnaires is much slower than biochemical recovery, and successfully treated CD patients continue to suffer physical and social dysfunction, physical and emotional problems, more pain, and decreased general well-being, all of which have a long-term residual effect on HRQoL.16–18
There are two significant factors in CD management after surgery: first, the condition of cure or remission and potential recurrence, and second, recovery of the hypothalamic–pituitary–adrenal axis, which may take longer than two years.
Three groups of patients may be distinguished after surgery: the patients easiest to identify are those with persistent hypercortisolism, while the other two groups are part of a spectrum ranging from eucortisolism to adrenal insufficiency.
The definition of recurrence is not clearly established either, because it poses the same problems as diagnosis. While some authors define recurrence as the presence of evidence of hypercortisolism, others define it as the absence of a response to suppression tests or even the loss of circadian rhythm with eucortisolism, or a combination of several criteria. This should be taken into account when interpreting recurrence rates, which are not comparable between the different series, which vary in turn in follow-up time and repeat surgery rates.19–21
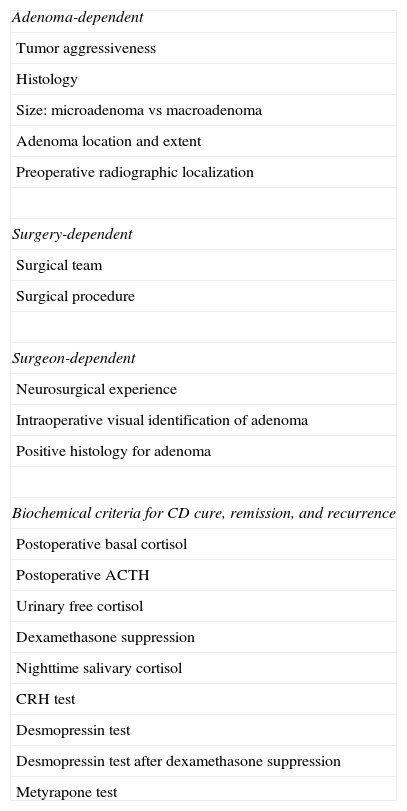
Remission predictors (Table 1)Adenoma-dependent factorsTumor size, extension, and aggressiveness are clearly related to the postoperative results. Obviously, more aggressive or invasive macroadenomas have a lower cure rate, while experienced neurosurgeons may achieve a 95% remission rate in microadenomas.9,22,23 Several studies show that immunohistochemical confirmation of the tumor correlates to a higher cure or remission rate, which is associated with the identification of adenomatous tissue by the neurosurgeon.24,25 Hyperplasia is rare and is associated with poorer results than adenoma because of the lack of identification of adenomatous tissue, which in some cases requires the performance of selective hemihypophysectomy.
Predictors of remission of Cushing's disease reported in the literature.
| Adenoma-dependent |
| Tumor aggressiveness |
| Histology |
| Size: microadenoma vs macroadenoma |
| Adenoma location and extent |
| Preoperative radiographic localization |
| Surgery-dependent |
| Surgical team |
| Surgical procedure |
| Surgeon-dependent |
| Neurosurgical experience |
| Intraoperative visual identification of adenoma |
| Positive histology for adenoma |
| Biochemical criteria for CD cure, remission, and recurrence |
| Postoperative basal cortisol |
| Postoperative ACTH |
| Urinary free cortisol |
| Dexamethasone suppression |
| Nighttime salivary cortisol |
| CRH test |
| Desmopressin test |
| Desmopressin test after dexamethasone suppression |
| Metyrapone test |
Other histological factors associated with a poor prognosis are the presence of Crooke cells (found in more aggressive tumors, most commonly macroadenomas)26 and the exceptional cases of ACTH-secreting carcinomas which do not cure after surgery in the presence of metastases.
Presurgical location using imaging techniques has also been reported to be a good prognostic factor. In some series, even macroadenomas have a higher remission rate than those with no prior image on MRI.27 In addition, some studies correlate the confirmation of gradient following inferior petrosal sinus sampling to the corticotropin-releasing hormone (CRH) test as a good predictor, although this will actually confirm diagnosis, and the results will depend on the neurosurgeon's experience in adenoma identification.28
Surgery-dependent predictorsThere is no doubt that the outcome of surgery, and thus the remission rate, directly depends on neurosurgical experience.5 The surgical procedure, and particularly the surgeon's experience with it, is also important. Articles advocating endoscopic surgery on the grounds that it provides results at least similar to microscopic surgery have recently been published,29 but personal experience with each of the procedures ensuring optimal results is ultimately the most important thing, and situations where the microscopic approach would be of choice have been reported.30 As discussed above, both the identification of adenomatous tissue by the surgeon during the procedure, which is possible even in cases where no MRI images are available, and the histological confirmation of adenoma by immunohistochemistry have been reported to be good predictors, but it should not be forgotten that most tumors are small and the collection of a tissue sample may be difficult, so that this criterion is not indispensable.
Results are worse when a patient requires repeat surgery due to disease persistence or recurrence.
Clinical predictorsThe need for glucocorticoid replacement therapy and its longer duration are significantly related to a lower recurrence rate.31,32 Most studies have shown that preoperative clinical variables such as sex, age, tumor size, gross tumor invasion, disease duration, and clinical symptoms are not significant predictors of recurrence. Presurgical hormone tests (preoperative urinary free cortisol or ACTH levels or DXM suppression tests) are also not helpful in this regard.33
Biochemical predictors of remission and recurrenceBasal plasma cortisol levelsPlasma cortisol levels less than 2mcg/dL in the first 48h after surgery have been reported to be associated with the long-term remission of CD. There is, however, no agreement in the literature on the cut-off point (<5, <2, <1.8, <1.3, and even <1mcg/dL) and the most adequate measurement time: 24–48h, 10–14 days (some studies suggest a greater accuracy when measurement is done two weeks as compared to two days after surgery), and even months after surgery.22,32,34–44
This criterion has a high positive predictive value, as shown in a recent study where 9/10 recurrences were associated with postoperative plasma cortisol levels higher than 50nmol/L (1.8mcg/dL), but does not rule out the possibility of recurrence during follow-up (up to 10% at 10 years), which is lower as compared to cases where this nadir was not achieved.5
In patients meeting the criteria, recurrence is more common in those with cyclic cortisol secretion before surgery (20%), who frequently have macroadenomas. It should not be forgotten that cyclicity may occur in more than 15% of patients. Cyclicity is underdiagnosed, and is not documented in the series.45
Other authors suggest that instead of a given cortisol level, the recovery of the normal functional characteristics of the hypothalamic–pituitary–adrenal axis (circadian rhythm, suppression after dexamethasone, and response to hypoglycemia) should be required, because recurrence is exceptional in these cases after normalization.46
The preoperative use of drugs decreasing cortisol secretion may derepress normal corticotroph cells leading to ACTH secretion. It is important to know if the patient has received drug treatment before surgery to control hypercortisolism (such as ketoconazole) and up to what time to interpret the results, and few series mention their use and the discontinuation period before surgery.23,40,42
The perioperative use of glucocorticoids inhibits ACTH secretion by any tumor cells that may remain in the tumor bed and induce the false inhibition of cortisol levels in subsequent retests, which may contribute to recurrence in some patients initially identified as cured because of the finding of plasma cortisol levels less than 2mcg/dL. Pituitary ACTH secretion is highly responsive to glucocorticoids, and even low doses may suppress ACTH release. In CD, at least 5% of patients have suppression of cortisol secretion with low-dose dexamethasone suppression. Several groups suggest cortisol monitoring in the early postoperative period without glucocorticoid replacement therapy provided the necessary means are available, which allows for knowing the surgical outcome and for avoiding the interference of glucocorticoid treatment with subsequent assessment.22,34,36,40,41
Although some authors advocate the use of postoperative basal cortisol levels also for the assessment of early repeat surgery, it should be noted that 5.6–20% of patients with clearly detectable basal plasma cortisol levels (higher than 5mcg/dL) may achieve complete clinical and biochemical remission (cortisol level less than 1.8mcg/dL) in 6–12 weeks, mostly in cases with macroadenoma, and early repeat surgery is therefore not recommended in patients with plasma cortisol levels less than 200nmol/L (7.2mcg/dL).21–23,36,44,47
Urinary free cortisol and suppression with dexamethasoneThey both have a lower prognostic value for remission as compared to postoperative basal cortisol. In a multicenter European study of 510 patients who achieved clinical and biochemical remission after transsphenoidal surgery, out of 65 patients with normal dexamethasone suppression test results after surgery, 12.7% experienced a recurrence of CD, as compared to a recurrence rate of 4.3% found in 94 patients with undetectable postoperative basal cortisol.31 In a North American cohort, 215 (85%) who achieved normalization of urinary free cortisol (UFC) had a 25% recurrence rate, while in 97 patients (45%) with postoperative basal cortisol levels less than 60nmol/L (2mcg/dL), the recurrence rate was 20% at five years.8
UFC may provide additional information when serum cortisol results are doubtful. Levels less than 20 mcg/24h suggest remission, while values within the normal range may be confounding and values above the normal limit suggest tumor persistence.5 Despite intra-subject variability, UFC continues to be of great value as a treatment target because its normalization is associated with significant improvement.
Nighttime salivary cortisolSeveral authors have shown the value of regular measurement of nighttime salivary cortisol in the postoperative follow-up of patients with CD with 90–100% sensitivity for detecting surgery failure and recurrence and 98% sensitivity when a cut-off point less than 2ng/mL is used. They therefore propose the use of this test because of its advantages over UFC, convenient sample collection, and lower intra-subject variability.48,49 However, unlike as occurs in CD diagnosis, there is no agreement regarding the criteria to be used to assess remission, which makes it difficult to compare the series.50
Unfortunately, this procedure is not available in many centers, and the technique should be validated for the results to be reliable.
Adrenocorticotropic hormoneACTH levels after surgery have been less well studied, although their prognostic value appears to be similar to that of basal cortisol. A cut-off point of ACTH 34pg/dL has been reported to have 80% sensitivity and 97.5% specificity for identifying patients in remission,34 while other authors report a cut-off value of <10–20pg/dL as a marker of adenoma resection.51 The adequate handling of samples is required, and a transient peak may occur after surgical handling.
Corticotropin releasing hormone testThis is based on the hypothesis that a normal ACTH response to the CRH tests after surgery may identify a subgroup of patients at a high risk of recurrence because it comes from incompletely resected abnormal corticotropic tissue. A normal or exaggerated cortisol or ACTH response to the CRH test has been reported to be a poor prognostic factor and a predictor of recurrence but without improving postoperative basal cortisol results.31,52,53
Desmopressin testThe use of the desmopressin test to detect a cure of CD is based on the loss of response of plasma cortisol and ACTH after the administration of desmopressin in patients with CD with a prior positive response to surgery, because healthy volunteers have a poor response to ACTH and cortisol following desmopressin administration. This test has a low sensitivity as a method for diagnosing CD, and a low sensitivity and positive predictive value when used as a prognostic remission factor.54–57
Dexamethasone suppression test after desmopressinThis test has been used in an attempt to improve the specificity of the desmopressin test because dexamethasone will theoretically suppress the secretion of normal corticotroph cells, but any corticotroph tumor cell may respond to desmopressin. Although ACTH response to this combined test has been associated with an increased risk of recurrence, the test has low specificity and positive predictive value.58
Postoperative glucocorticoid use should be taken into account when interpreting these last two tests.
Metyrapone testThe inability to increase ACTH secretion and subsequently 11-deoxycortisol secretion following the administration of metyrapone, which blocks 11-beta-hydroxilase, may suggest complete adenoma resection.59 However, using the metyrapone test provides little advantage over the use of plasma basal cortisol as a remission predictor.60
Our own experience. Preliminary results (Table 2)Since 2005, the management approach used for patients with Cushing's disease undergoing transsphenoidal surgery at Hospital Universitario de La Ribera has consisted of the prior discontinuation of suppressing treatment (at least one week before admission), no perioperative corticoid use, and the monitoring of ACTH and cortisol levels in the early postoperative period. Data were prospectively collected in order to conduct a study that would allow for assessing their value as predictors of long-term disease remission.
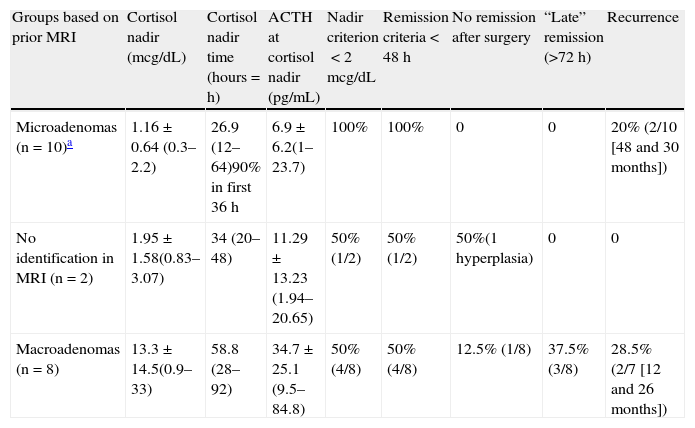
Mean cortisol nadir and ACTH values after transsphenoidal surgery and remission/recurrence criteria after transsphenoidal surgery in Cushing's disease (preliminary results at Hospital Universitario de La Ribera).
| Groups based on prior MRI | Cortisol nadir (mcg/dL) | Cortisol nadir time (hours=h) | ACTH at cortisol nadir (pg/mL) | Nadir criterion<2mcg/dL | Remission criteria<48h | No remission after surgery | “Late” remission (>72h) | Recurrence |
| Microadenomas (n=10)a | 1.16±0.64 (0.3–2.2) | 26.9 (12–64)90% in first 36h | 6.9±6.2(1–23.7) | 100% | 100% | 0 | 0 | 20% (2/10 [48 and 30 months]) |
| No identification in MRI (n=2) | 1.95±1.58(0.83–3.07) | 34 (20–48) | 11.29±13.23 (1.94–20.65) | 50% (1/2) | 50% (1/2) | 50%(1 hyperplasia) | 0 | 0 |
| Macroadenomas (n=8) | 13.3±14.5(0.9–33) | 58.8 (28–92) | 34.7±25.1 (9.5–84.8) | 50% (4/8) | 50% (4/8) | 12.5% (1/8) | 37.5% (3/8) | 28.5% (2/7 [12 and 26 months]) |
Mean values: mean±standard deviation (range).
Mean follow-up time: 52.8 months (37–76 months).
Preliminary data from the first 20 patients in the series (15 females and 5 males), with a mean age of 43.1 years (17–63 years), who underwent surgery from December 2005 to March 2009 are reported. Mean follow-up time was 52.8 months (37–76 months). Surgery was performed through a transnasal transsphenoidal approach in all cases. ACTH and cortisol levels were measured every 4–6hours during the first 72h after surgery (or until cortisol levels <2mcg/dL were achieved). Patients were admitted to the ICU during this postoperative period, and their clinical signs were monitored.
Nineteen patients underwent selective resection of pituitary adenoma. A patient with no tumor image in the preoperative MRI and no intraoperative identification of tumor tissue underwent hemihypophysectomy guided by the results of petrosal sinus catheterization. Immunohistochemistry was positive for ATCH in all of them. After surgery, 15 patients (75%) developed adrenal insufficiency during the monitoring period: 100% of patients with microadenomas (10/10), 50% of those with macroadenomas (4/8), and 50% (1/2) of patients with no tumor image. Three patients with macroadenoma who did not meet this criterion in the early postoperative period also achieved late clinical and biochemical remission. No significant clinical complications occurred during the monitoring period as the result of a lack of use of corticoid replacement.
Nine months after surgery, all patients in whom secondary adrenal insufficiency had occurred in the early postoperative period met disease remission criteria. A recurrence of Cushing's disease occurred in four patients (20%): two with microadenomas (30 and 48 months after surgery respectively) and two with macroadenoma (after 12 and 26 months respectively). In both the patient with macroadenoma with persistent disease after surgery and the two patients with recurrence during follow-up, cavernous sinus infiltration (at least Knosp grade 2) was seen in preoperative MRI. Patients with persistent remission required longer replacement therapy (>6 months).
These preliminary results lead us to conclude that the postoperative management of patients undergoing surgery for Cushing's disease without the use of corticoid replacement therapy and with the monitoring of ACTH and cortisol levels is safe. A cortisol nadir less than 2mcg/dL after surgery was helpful as a predictor for long-term disease remission in patients with microadenoma. It is also suggested that in microadenomas and in cases with no preoperative imaging diagnosis, the identification and selective excision during surgery of tissue with an adenomatous appearance may be correlated to a greater chance of long-term disease remission (a result which was achieved in all patients in our series where such identification was possible). A failure to achieve this nadir during the monitoring period in macroadenomas did not necessarily represent a persistence of active disease, because 75% (3/4) of patients who did not achieve these levels in the monitoring period did subsequently meet disease remission criteria. Finally, the recurrence rate (20%) was similar to that reported in other previously published series.
ConclusionNo agreement exists on the definition of the criteria for cure or remission, or even recurrence, in CD. Neurosurgical experience is essential to achieve good results. As regards the biochemical prognostic factors, it is well documented in the literature that an undetectable postoperative cortisol nadir, at least lower than 2mcg/dL, predicts for disease remission, but recurrence cannot be ruled out even in these cases, and lifetime follow-up is therefore required in these patients. Among the clinical parameters, the need for corticoid replacement therapy after the undetectable nadir is achieved, and particularly its duration, is the only one which has been shown to be related to a higher remission rate.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Abellán Galiana P, Fajardo Montañana C, Riesgo Suárez PA, Gómez Vela J, Meseguer Escrivá C, Rovira Lillo V. Factores pronósticos de remisión a largo plazo tras cirugía transesfenoidal en la enfermedad de Cushing. Endocrinol Nutr. 2013;60:475–482.