Thyroid orbitopathy is the most prevalent non-thyroid symptom in Graves’ syndrome. It has a high incidence and particularly affects young women. Smoking is clearly involved in its development and progress, and in its response to different treatments. This autoimmune condition usually has a benign course, independent from hyperthyroidism, but its severe, progressive forms represent a major therapeutic challenge. Clinical evaluation poses great difficulties, as there is no truly objective rating scale representing disease activity. New molecular or inflammation markers may prove to be useful in this regard. This review reports new findings about its pathophysiology and the different techniques used for treatment over time. Discussion particularly focuses on the immunomodulatory role of radiotherapy, as well as on its role together with corticosteroids.
La orbitopatía tiroidea es el síntoma no tiroideo más prevalente en el síndrome de Graves. Presenta una alta incidencia, afectando especialmente a mujeres jóvenes. Existe una clara relación causal con el tabaco, que se halla implicado tanto en su desarrollo como en su evolución y en la respuesta a los diferentes tratamientos. Se trata de un proceso autoinmune que suele evolucionar de manera benigna e independiente del hipertiroidismo, aunque cuando es grave y progresiva representa un gran reto terapéutico. Su evaluación clínica presenta grandes dificultades al no existir una escala de valoración realmente objetiva y representativa de la actividad de la enfermedad. En esta línea pueden ser útiles nuevos marcadores moleculares o de inflamación. En la presente revisión se describen nuevos hallazgos sobre su fisiopatología, así como las diferentes técnicas utilizadas para su tratamiento a lo largo del tiempo. La discusión se centra especialemente en el papel inmunomodulador de la radioterapia, así como en su papel junto con los corticoides.
Graves’ or Basedow's syndrome is characterized by the classical triad of hyperthyroidism with diffuse goiter, orbitopathy, and dermopathy. Its symptoms may occur together during the course of the disease or isolated and separately.
The incidence of orbital involvement in patients with Graves’ syndrome is estimated at 25–50%. Involvement may range from mild manifestations to major symptoms. Only 5% of patients with thyroid orbitopathy(TO) require more aggressive treatment, such as glucocorticoids or radiotherapy. The incidence of some grade of orbitopathy in Graves’ syndrome in the general population is approximately 16 women and 3 men per 100,000 population, with a female:male ratio of 5:1.1,2 TO usually starts between the third and fourth decades of life. Its clinical presentation may be more severe in elderly subjects and men, and milder in Asian people.2,3
Smoking is clearly related to TO development and course, and also to its response to and reactivation after treatment.4–6 This relationship was suggested from the first description of TO, and is somewhat greater in women. The link between smoking and orbitopayhy could be due to tissue hypoxia, cytokine-mediated modulation, and the enhancement in HLA-DR expression by fibroblasts. Smoking cessation is one of the mainstays of treatment for this disease.7
Genetic factors play an essential role; an increased frequency of haplotypes HLA B8, DRw3, Bw36, Bw46 and the single nucleotide polymorphism (SNP) rs179247 is found, suggesting a clear familial predisposition.8
TO may affect one or both eyes. Bilateral TO is most common, although the severity of the condition in each orbit may be different. Clinical signs and symptoms occur gradually, and there is usually a weak correlation with the severity and duration of thyroid dysfunction. From 80% to 90% of patients have hyperthyroidism during TO onset.2 As a result of thyroid treatment, TO may improve in 50–64% of patients or stabilize in 22–33%.9,10 Although ocular manifestations are associated with thyrotoxicosis, the possibility of intraorbital or intracranial disease should be ruled out. This requires differential diagnosis with cavernous sinus thrombosis, sphenoid wing meningioma, retrobulbar and intracranial tumors (including orbital lymphoma), idiopathic orbital inflammatory disease or eye pseudotumor, lymphoid hyperplasia, uremia, malignant hypertension, chronic alcoholism, chronic obstructive pulmonary disease, superior mediastinal obstruction, carotid-cavernous fistula, and Cushing's syndrome.
If doubt exists about TO etiology, the detection of significant titers of thyroid-stimulating immunoglobulins, immunoglobulins inhibiting TSH binding, peroxidase antibodies, abnormal stimulation with thyrotropin-releasing hormone, or thyroid suppression tests suggest TO. It should be noted that there are euthyroid and even hypothyroid patients with TO who show no changes in these markers.2
Orbitopathy, when severe and progressive, is the component of TO most difficult to treat. TO most often has a benign course independent of hyperthyroidism, and may even be self-limited over time in moderate to severe cases, with only some degree of exophthalmos and ophthalmoplegia.2,9 When treatment has been decided, the fact that peak activity occurs between 13 and 24 months after disease onset should be taken into account. Most patients (up to 80–90%) may subsequently improve or remain stable.11 By contrast, up to 5% may experience a late reactivation.2
It has not been confirmed that either complete thyroid ablation by surgery or metabolic therapy with iodine-131 is more beneficial for ophthalmic disease than the use of antithyroid drugs. There is however agreement as to the need for adequate thyroid function monitoring.7,12
PathophysiologySignificant advances have recently been made in our understanding of the pathophysiology of TO. Two processes of special interest occur in this regard: the development of T cells reactive to the thyrotropin receptor (TSH-R) and the establishment of a complex network of cytokine-mediated cell interactions in which multiple immune system cells are involved.
There is an initial failure in immune control that allows for the development of autoimmunity to TSH-R. In this regard, a SNP, rs179247, has been found in intron 1 of the TSH-R gene8 When tolerance is induced in the thymus gland by eliminating all T cells reactive to self antigens (95% of all T cells), including TSH-R, the presence of such polymorphism induces a low TSH-R expression that prevents its adequate antigen recognition and the resultant suppression of the lymphocyte population that recognizes it. However, those same lymphocytes may recognize TSH-R at a later time, once they are in the systemic circulation.8
The immune process continues with TSH-R internalization and degradation by antigen-presenting cells. These cells present the resulting peptides, together with the major histocompatibility complex class II (CMHII), to T-helper cells (THCs), which are activated by interacting with self-reactive B cells. THCs secrete interleukin-2 (IL-2) and interferon gamma (IFγ). These cytokines induce the differentiation of B cells into plasma cells, which in turn produce anti-TSH-R antibodies (anti-TSH-R Ab). In the thyroid gland, these antibodies stimulate TSH-R in follicular epithelial cells, causing hyperplasia and the production of thyroid hormones, triiodothyronine (T3), and thyroxine (T4).
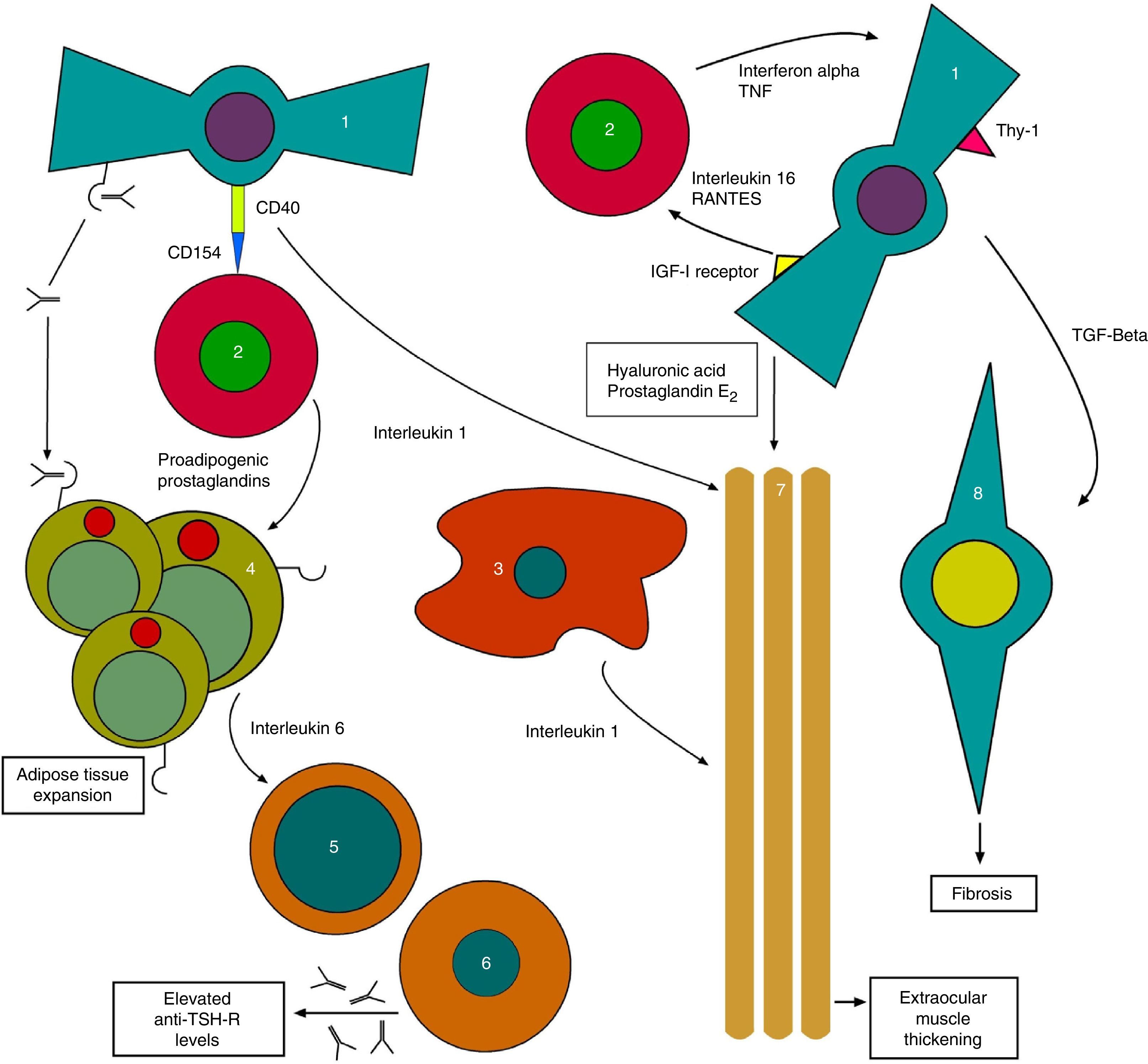
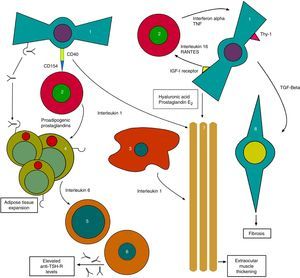
On the other hand, anti-TSH-R Ab also acts upon TSH-R in orbital fibroblasts, which together with the substances released by THCs generate the tissue changes characteristic of TO with the expansion of orbital tissues13 (Fig. 1).
Initial pathophysiological mechanism of thyroid orbitopathy. 1: orbital fibroblast; 2: T-helper cell; 3: macrophage; 4: orbital adipocyte; 5: B cell; 6: plasma cell; 7: extraocular muscles; 8: myofibroblast.
Based on: Bahn15.
The process continues with stimulation by anti-TSH-R of fibroblasts called preadipocytes, which differentiate into adipocytes with a high TSH-R expression. Meanwhile, other fibroblasts bearing antigen Thy1 (FPA-Thy1) are stimulated by cytokines TNF (tumor necrosis factor) and IFγ, and increase their production of hyaluronic acid, which they release into the environment.
In addition, the insulin-like growth factor receptor (IGF1-R) expressed in orbital fibroblasts is also stimulated, with the production of IL-16 and the regulated upon activation, normal T-cell expressed and secreted (RANTES) protein, which in turn stimulate the recruitment of THCs and other mononuclear cells in the orbit, providing feedback, and promoting the orbital adipogenesis present in orbitopathy.14
Direct interconnections also exist between THCs and fibroblasts, generating the joint production of IL-1. IL-1 is also released by macrophages, which combined with TNF and IFγ released by THCs further stimulate fibroblasts, which in turn release hyaluronic acid and prostaglandin E2 (PGE2). Hyaluronic acid, which is highly hydrophilic, accumulates in the orbital space around the extraocular muscles and between adipocytes. All of these lead to a widening of orbital tissues.
Cycle feedback continues when THCs produce proadipogenic prostaglandins that stimulate preadipocytes, thus contributing to exophthalmos by volume occupation.
Adipocytes also participate by generating, together with fibroblasts, IL-6 which in turn causes the maturation of B cells, increasing their production of anti-TSH-R Ab. Finally, fibroblasts also produce transforming growth factor beta (TGF-β) that stimulates both hyaluronic acid production and the transformation of FPA-Thy1 into myofibroblasts, which participate in the development of fibrosis, especially in advanced stages of the disease.15
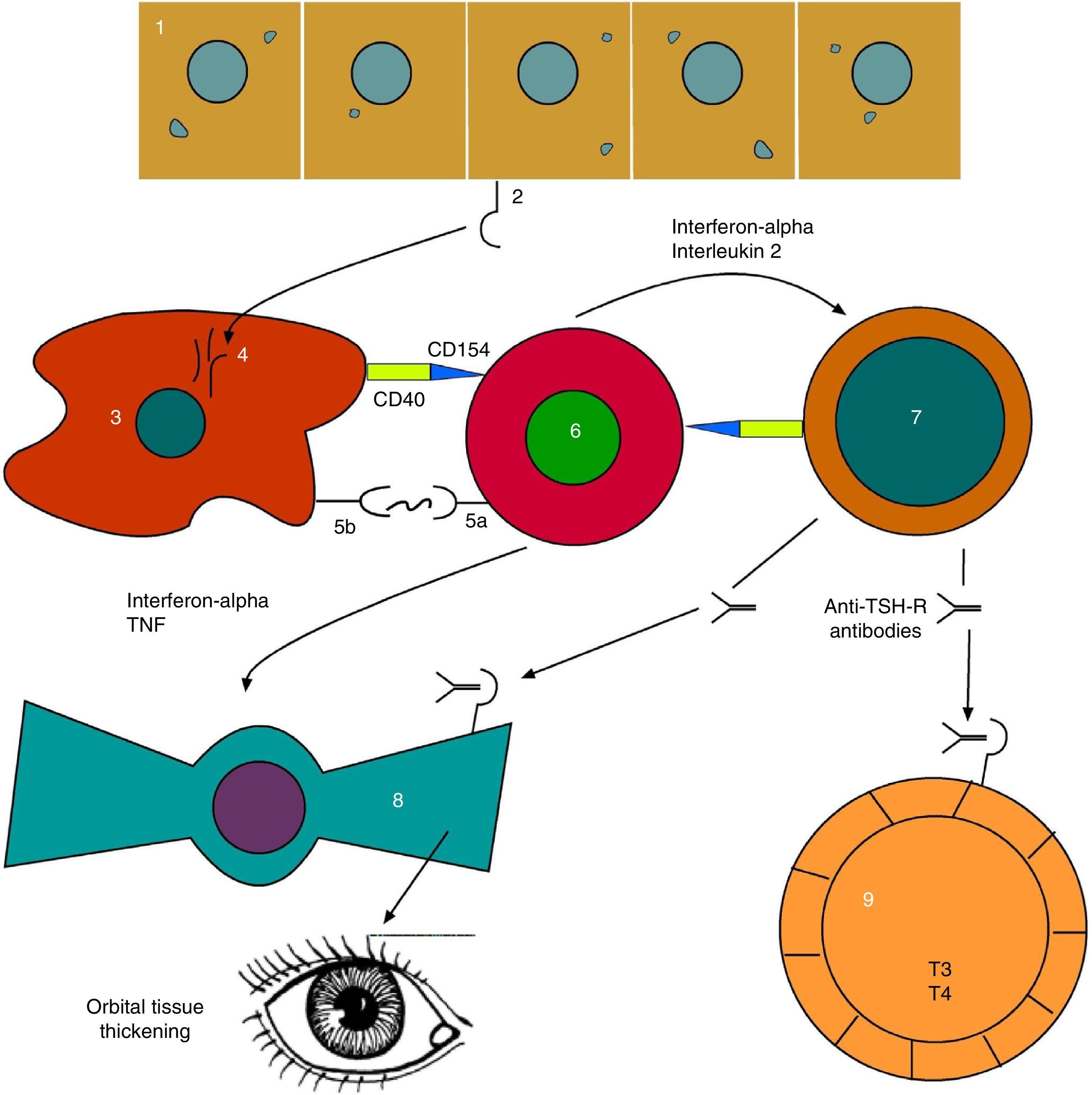
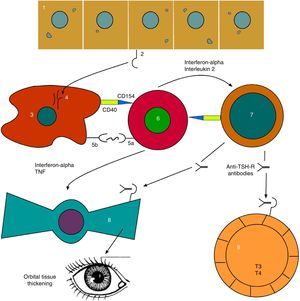
As can be seen, advances have been made in our understanding of the complex network of autocrine and paracrine signals which allow for communication among the different cells involved (Fig. 2).
Evolved pathophysiological mechanism of thyroid orbitopathy. 1: thyroid follicular endothelial cell; 2: TSH receptor (TSH-R); 3: antigen-presenting cell; 4: TSH-R degradation peptides; 5a: T-cell receptor; 5b: CMH class II; 6: T-helper cell; 7: B cell; 8: orbital fibroblast; 9: thyroid hyperplastic follicle.
Based on: Bahn.15
All signs and symptoms result from an inflammatory/infiltrative process with congestive events:
Signs- ∘
Lid retraction.
- ∘
Slow lid movements.
- ∘
Restriction of eye movements with limitation of superior gaze and convergence insufficiency.
- ∘
Lid and conjunctival hyperemia.
- ∘
Lid edema.
- ∘
Conjunctival and caruncular chemosis.
- ∘
Proptosis/exophthalmos.
- ∘
Ptosis.
- ∘
Corneal opacity.
- ∘
Papilledema.
- ∘
Lagophthalmos.
- ∘
Corneal ulcers.
- ∘
Visual deficits.
- ∘
Altered color perception.
- ∘
Diplopia (initially intermittent).
- ∘
Blurred vision.
- ∘
Hypersensitivity to light.
- ∘
Pain on eye movements.
- ∘
Eye pain or retro-ocular pain at rest.
- ∘
Sensation of change of image.
There are multiple measurement scales focusing on different aspects of TO, many of which do not adequately reflect the degree of activity. The lack of a really objective assessment may contribute to the myriad disparate results in studies reported to date. We should add to this the great clinical variability, the trend to spontaneous improvement, and factors that modulate the response to treatment such as smoking, age, sex, diabetes mellitus, thyroid status, prior treatments, or long-standing diseases.11
Adequate, widely accepted measurement scales are required for the correct interpretation of treatment efficacy. Truly objective and reproducible assessment methods are currently lacking. New biological or inflammation markers, and even the use of magnetic resonance imaging, may help to improve TO diagnosis and grading.16–18
The scales used include:
- •
ENOSPECS: divided into classes and grades, it is the scale most widely used in the US. It is subjective, difficult to complete, and requires prior training.19
- •
The Clinical Activity Score (ECAS): this is the most widely used scale in Spain and Europe. It assesses pain and inflammatory findings in soft tissues. It has been shown to be effective for assessing patient response to both corticosteroid treatment and radiotherapy.20 According to Gorman, some items on this scale (periorbital and caruncle edema and chemosis) are difficult to define because their cause may be congestive rather than inflammatory, as both processes are closely related.1,11 Although it is more objective, doubts have been raised with regard to treatment decisions in patients with Grade 2–3.
- •
Other assessment scales: VISA, EUGOGO, OI, STI, EMR, GAG, MRI, Octreoscan.21,22
- •
Quality of life assessment scales23:
- ∘
General: MOS SF-24, and SF-36.
- ∘
Specific: GO-QoL.
- ∘
- •
Symptomatic measures indicated in the initial stage of the disease include eye lubrication, light protection, cold packs, elevation of the bed head, lid occlusion at night, botulinum toxin in the event of upper lid retraction, and prisms if diplopia exists.11,24
- •
Smoking cessation should also be among the essential therapeutic actions in TO.4–7
- •
There is agreement on the importance of controlling hyperthyroidism either through anti-thyroid drugs or any other type of thyroid ablation,15 but it has been demonstrated that anti-thyroid drugs and thyroidectomy do not change the course of the disease, while treatment with iodine-131 may worsen the condition.25 The use of prophylactic low-dose corticosteroids is recommended after ablation with iodine-131, particularly in active smokers, to prevent TO progression during radioablation.7,25,26
- •
Corticosteroid therapy has been used for a long time to treat TO using different schemes and administration routes. The objective is to decrease the inflammatory-infiltrative component. Symptom control has been reported in up to 65–85% of patients.27,28
The systemic route, using an intravenous bolus, is the most commonly used today. The drugs used are methylprednisolone and prednisone.27–29 High doses are used, with tapering to the minimum effective dose and long-term maintenance. The dosage scheme most widely used in the United States and Europe consists of methylprednisolone 500mg IV weekly for 6 weeks, followed by 250mg IV weekly for an additional 6 weeks. If the patient has not responded after this time, the use of other therapies should be considered. Higher doses have only shown a somewhat greater response in the short term, associated with increased toxicity.30 Before administration, adequate patient selection, with any comorbidities being taken into consideration, and appropriate liver assessment are recommended.27 If no compression neuropathy exists, it is advisable not to exceed a cumulative dose of 8g.27,31
Systemic corticosteroid therapy has been shown to be more effective than retrobulbar injection.32 On the other hand, the evidence from randomized trials and two meta-analyses shows greater efficacy and less iatrogeny of glucocorticoids as an intravenous bolus as compared to the administration of high, sustained oral doses.27,28,33–35
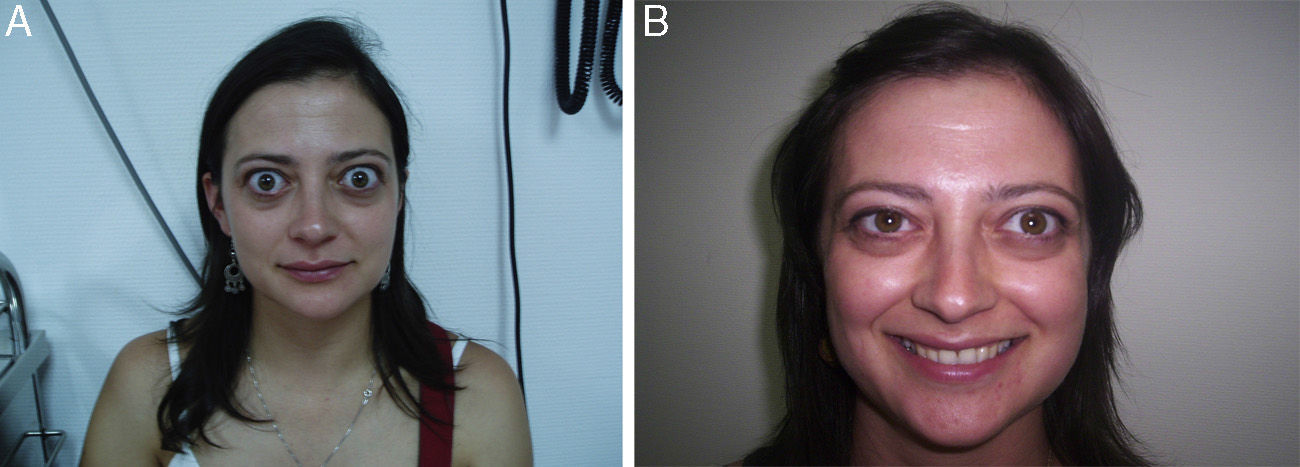
After the discontinuation of corticosteroid therapy, the reactivation of orbital clinical signs usually occurs in a high proportion of patients.30,36 Specifically, a study by the European Group on Graves’ Orbitopathy (EUGOGO) on three different cumulative dose schemes of intravenous methylprednisolone to treat moderate to severe TO reported 24-week recurrence rates after an initial improvement of 33%, 21%, and 40% with doses of 7.47, 4.98, and 2.25g respectively30 (Fig. 3).
Finally, the side effects of long-term corticosteroid therapy should be taken into account. Such effects include hyperglycemia, high blood pressure, immune compromise, predominantly proximal myopathy, capillary fragility, fat distribution remodeling, skin rash, psychiatric disorders, osteoporosis, liver disease, etc., including fatal events.27,31
Surgical orbital decompression should be reserved for rapidly progressing compressive optic neuropathy that does not respond to corticosteroid treatment and radiotherapy.37 It is also indicated for patients with no physical activity with severe functional or cosmetic sequelae37 after a reasonable period of 6–12 months have elapsed and provided they are euthyroid.24 Surgical decompression consists of resection, using different approaches, of part of the orbital bone to relieve intraorbital pressure. The transpalpebral and transconjunctival approaches are most commonly used.37,38 The removal of orbital fat alone has been shown to be less effective, with a high recurrence rate.39 A marked reduction of exophthalmos/proptosis is achieved in the inactive stages of the disease. Decompression has been reported to be effective and safe when progression occurs after medical treatment and radiotherapy, or for resolving strabismus due to established muscle fibrosis.37,40 It should also be noted that this procedure may worsen prior strabismus, a condition difficult to resolve.
Other treatments, such as azathioprine and metronidazole, have been used with no satisfactory results. Topical 5–10% guanethidine sulfate has also been used with little efficacy and undesirable side effects including punctate keratitis and irritation in the instilled area.41 Recent randomized, placebo-controlled studies which assessed the role of octreotide as an inhibitor of lymphocyte proliferation and activation found no efficacy.21,42
In recent years, as the result of greater understanding of the pathophysiology, new drugs targeted to many new therapeutic targets have been evaluated.15 Tocilizumab43 and rituximab44 have shown particularly promising results.
A recent randomized, double-blind study45 comparing a selenium supplement (100μg twice daily) for six months with pentoxifylline (600mg twice daily) and placebo (twice daily) in patients with mild orbitopathy showed improvements in quality of life and eye involvement, as well as decreased TO progression, in patients treated with selenium, but not in those given pentoxyfilline. These effects were evident between 6 and 12 months of follow-up. No adverse effects were seen with selenium, but gastrointestinal problems occurred with pentoxyfilline. It should be noted that the long-term use of selenium is associated with a greater risk of the development of type 2 DM (with doses of 200μg daily), peripheral vascular disease, all-cause mortality, and glaucoma. A potential interaction may also occur with antiaggregants and anticoagulants.46,47 Selenium should therefore not be used for longer than six months. Interestingly, an Australian study showed lower serum selenium levels in patients with TO.48
RadiotherapyRadiotherapy/RT) has been routinely used in TO since the 1940s, and so a lot of experience in its use has been accumulated. We have to go as far back as 1913 to find the first report of the use of radiotherapy,49 but it was not until the studies reported by Donaldson in 1973, with the generalization of megavoltage (linear accelerators and cobalt pumps), that the RT scheme used to date in most hospitals was established.50 This scheme consists of a total dose of 20Gy, divided into 2Gy fractions given five times weekly (normofractionation).
The initial indication of radiotherapy was empirical.32,36,51–53 The first randomized trial was reported in 1993.54 Meta-analyses supporting RT efficacy are currently available.55–58 The conclusions drawn from a literature review are given below. The levels of evidence and grades of recommendation used to describe RT efficacy are based on the criteria of the U.S. Preventive Services Task Force (USPSTF) updated in 2001.59
Indications of radiotherapy- 1.
There is no place for RT in the merely cosmetic treatment of TO.11
- 2.
RT is not indicated in mildly active disease.
- 3.
RT is indicated for the treatment of moderate to severe TO.32,34,55–58
- 4.
Compression neuropathy is an indication for high-dose corticosteroids and subsequent RT, because surgery is very difficult at this stage.60 However, if neural compression is not resolved with this approach, surgical decompression should be performed.11
- 5.
RT is also indicated after surgical decompression with incomplete resolution of clinical signs.16
Contraindications of RT: prior retinopathy or poorly controlled diabetes.61
Efficacy- 1.
Retrospective studies and meta-analyses have shown RT to be effective for controlling inflammatory signs in soft tissue (erythema, chemosis, periorbital edema), with response rates of approximately 80%. It is also effective for the recovery of vision lost due to compression in the active stages of the disease (41–71%). RT also acts to recover the mobility of the extraocular muscles affected (61%). Finally, remission of proptosis is uncommon (23–51% of patients), with responses of only a few millimeters and minimal or doubtful cosmetic impact.6,36,52,53,55,57,58,62–65Level of evidence I, grade of recommendation B.
- 2.
In a large retrospective study, the concomitant use of corticosteroid therapy and RT achieved a significant decrease in the risk of the development of optic neuropathy as compared to the use of corticosteroid therapy alone.62 Recent meta-analyses also support such efficacy.55,57Level of evidence I, grade of recommendation B.
- 3.
At least five systematic reviews of the efficacy of RT for the treatment of TO are available, including a review by the Cochrane Collaboration.55–58 It should be noted that many randomized studies contain a number of methodological biases that reduce the possibility of unequivocal conclusions based on such reviews from being drawn. The disease course itself, patient selection criteria, the measurement of different objectives focusing on activity assessment scales rather than on quality of life, etc., all make performing these reviews and achieving clear recommendations difficult. The overall conclusions regarding RT efficacy which can be drawn from these reviews are as follows:
- •
Greater efficacy with RT at 20Gy plus glucocorticoids is seen as compared to glucocorticoids alone.
- •
Greater efficacy with RT at 20Gy is seen as compared to simulated RT.
- •
Greater efficacy with RT at 20Gy plus intravenous glucocorticoids is seen as compared to RT 20Gy plus oral glucocorticoids.
- •
There is no difference between RT at 20Gy and glucocorticoids alone.
- •
There is no evidence that RT plus intravenous glucocorticoids is more effective than intravenous glucocorticoids alone.
- •
Finally, no differences are seen in quality of life, intraocular pressure, or costs with RT as compared to corticosteroid therapy. Level of evidence I, grade of recommendation B.
- •
- 4.
According to the reported series, once RT concurrent with corticosteroid therapy is completed, total and often indefinite discontinuation of corticosteroid therapy is possible in a high proportion of patients (from 71% to 90%).51–53Level of evidence II-III, grade of recommendation B.
- 5.
It is estimated that rescue decompression surgery is required in approximately 20% of cases after RT with or without corticosteroid therapy, largely because of persistent smoking.6,37Level of evidence III, grade of recommendation B.
- 6.
The more established the inflammatory-fibrotic process and the more evolved the orbitopathy, the lower the efficacy of RT.11–58Level of evidence I, grade of recommendation B.
- 7.
Improvement after RT sometimes occurs after a delay (>6 months).50Level of evidence II-III, grade of recommendation B.
- 8.
Finally, repeat RT achieves good response rates despite initial resistance or after symptom recurrence, with no apparent increase in morbidity.53Level of evidence II-III, grade of recommendation B.
Retrospective studies found no difference between RT doses of 20 and 30Gy given as fractions of 2Gy/session.51 Only two randomized studies comparing total dose (TD) are available. One of them compared 2.4Gy and 16Gy, at 0.3 and 2Gy/session respectively, and found no significant differences between the two fractionations.66 The other study compared three arms treated with 20Gy (1Gy/week), 10Gy (1Gy/day), and 20Gy (2Gy/day), the latter two in two weeks. A statistically significant improvement was seen in the first arm, as compared to the other two, in eye symptoms, palpebral fissure, intraocular pressure, proptosis, visual acuity, eye mobility, muscle thickening, and NOSPECS scale parameters. Lower radiation-induced toxicity and greater satisfaction were also recorded in patients on the first treatment Scheme.60 However, although this was a well balanced study, it was conducted on a small patient sample. Finally, according to a recent meta-analysis, no differences exist between RT at 20Gy and other RT schemes.55
Side effectsNo severe complications have been reported with RT, except for visual changes or blindness in the setting of prior diabetic retinopathy. RT is therefore contraindicated if there is prior retinopathy or poorly controlled diabetes.61 Cataract is the most common major side effect, but it may also be due to prior treatment with high corticosteroid doses.6,63
Some patients experience alopecia of the tail of the eyebrows due to inclusion in the radiation fields, with subsequent full recovery. Some eye dryness may occur in a very small proportion of patients.6,53,63
Long-term follow-up has not revealed a high number of radiation-induced second tumors.1,67 Some exceptional cases of pigmented basal cell carcinoma have been reported.68 The theoretical risk estimated after retrobulbar irradiation with 20Gy ranges from 0.3% to 1.2%.69 Hence, some authors recommend that young patients (<35 years) should not be treated.24
Follow-up for at least three years after RT completion is recommended, but there are series with longer follow-up times where no relevant toxicity has been shown.6,36,53,61,63,67 Surgical correction is often required for diplopia occurring after RT due to the effects on muscles or lids of the disease itself and its treatment.24,37,53 Surgery is usually performed soon after RT completion. A multidisciplinary approach to this complex condition, involving all the specialists concerned, is therefore required.
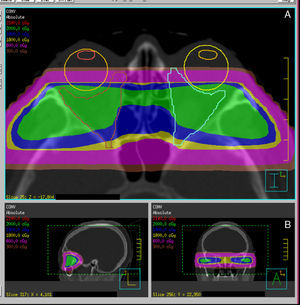
Radiotherapy procedureRadiation is normally performed using high energy photons (4–6MV). The patient is immobilized using a thermoplastic mask, and laser reference points are marked on the mask to allow for the reproduction of positioning during treatment sessions.
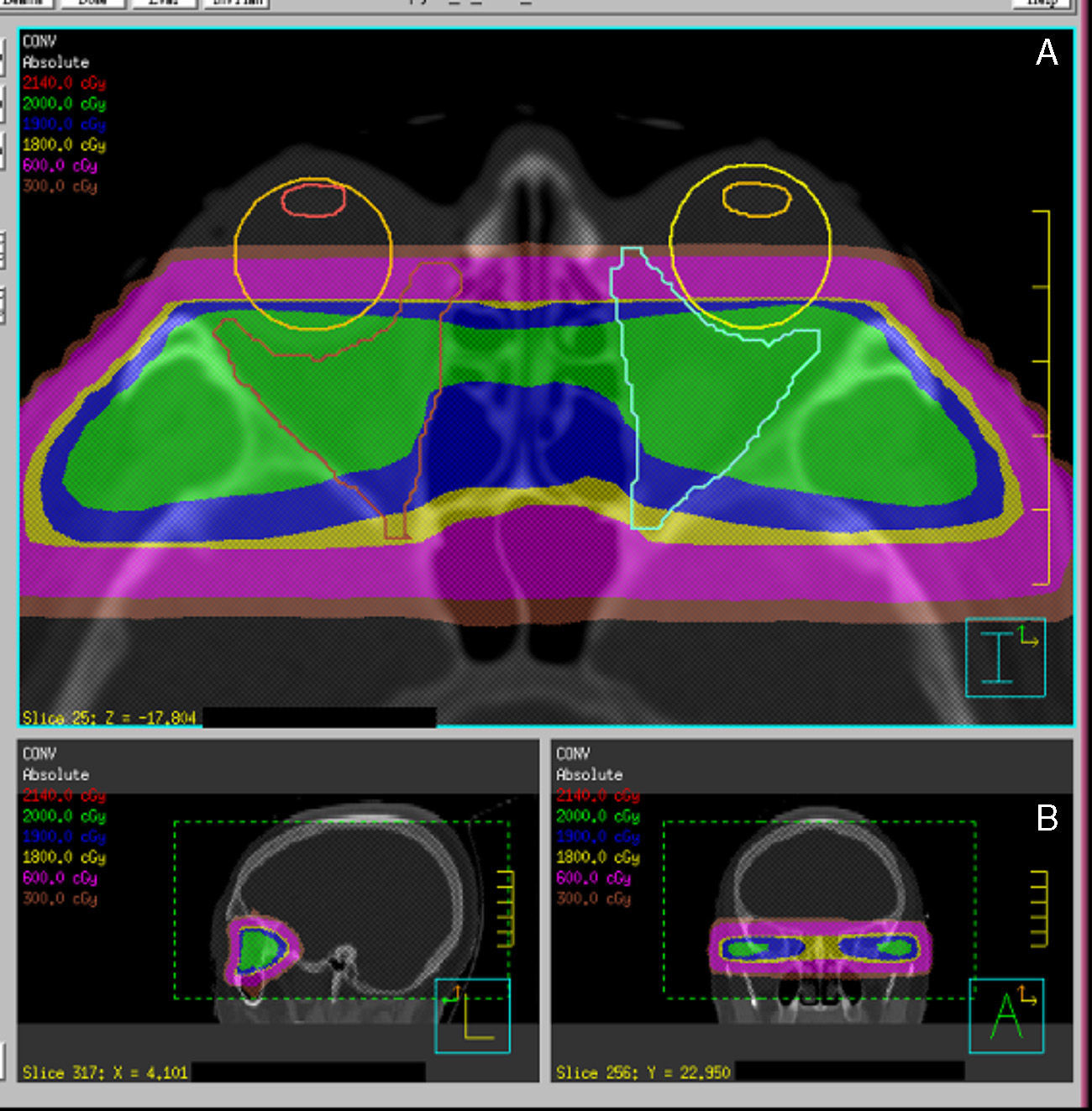
Procedures for more precise and conformed radiation localization and administration are currently available. Different RT systems are available, ranging from fractionated stereotactic RT to image-guided RT, and also including intensity-modulated RT and, an evolution of the latter, volumetric modulated arc therapy (VMAT). These are systems for dose localization, calculation, and administration which allow for improving the conformation of target volume while sparing organs at risk, in this case the lids, lens, retina, and pituitary gland (Fig. 4).
In conclusion, RT, with or without glucocorticoids, induces in moderate to severe TO significant improvements in the degree of diplopia, visual acuity, optic neuropathy, palpebral thickening, and eye mobility, although it is more effective when used concomitantly with corticosteroids. However, RT has not shown advantages over other treatment alternatives in terms of costs, intraocular pressure decrease, or quality of life.55–58Level of evidence I, grade of recommendation B.
DiscussionBecause of the conflicting results reported for the different treatments used in the different publications, it may be questioned whether the study samples were representative of patients with the activity required for immunosuppressant measures to be effective. It should be noted that both corticosteroid therapy and RT, and new treatment approaches, base their impact on an immunomodulatory action. In addition, the concomitant use of corticosteroids and RT has an added advantage, because corticosteroids control both the inflammation inherent to the disease and the edema induced by normofractionated RT.11,65 In any case, if low radiation doses (≤1Gy/session) are repeatedly used, concomitant corticosteroid therapy can be avoided. Such doses have been seen to be sufficient to relieve or control inflammatory symptoms by inhibiting the nitric oxide pathway present in such processes. This pathway is activated by the usual fractions of 2Gy/session, causing radiation-induced edema.60,70 Various institutions have used schemes with lower doses per session and longer total treatment times, with great efficacy in clinical control and less toxicity as compared to normofraccionation.66,71,72
Based on the foregoing, it may be summarized that patients with active (moderate to severe), short-lasting orbitopathy are those who may actually benefit from radiation.55,60,64 RT may be used both as first line treatment with or without concomitant corticosteroids and after the failure of, refusal of, or intolerance to intravenous corticosteroid therapy. Adequate evidence is available to support the use of RT over other alternatives, with similar or lower costs and the same quality of life. The side effects of RT are local and mild to moderate in severity. No fatal events have been related to its use. Radiation-induced tumors are exceptional, and the few cases reported of the development of retinopathy or optic neuropathy were usually related to prior, poorly controlled diabetes or to compression secondary to increased intraorbital pressure. However, close long-term monitoring is required in these patients.
On the other hand, there is no reliable way to measure disease activity, and all attempts at quantification appear to create more confusion, especially if differences between the observers are taken into consideration. Because of this, the use of new molecular or inflammation markers and magnetic resonance imaging may represent an advance in this regard.
An additional question concerns the time necessary to perform treatment, because satisfactory results will not be achieved in patients with long-standing disease, no inflammatory activity, and established fibrotic events. Due to the complexity of TO, a multidisciplinary approach involving all the relevant specialists is strongly recommended to ensure an improved quality of care.
Finally, patients and healthcare providers may have different expectations. This is why quality of life assessment should be a daily tool in the clinical management of these patients. It can provide objective information about the efficacy of the treatment of disease symptoms, not only of its signs. Thus, more objective, truly reliable parameters of the activity of orbitopathy that allow for adequate prescription of any immunosuppressant treatment, including RT, need to be described. Only then we will be able to decide if treatment is effective and if it is warranted after global consideration of all its aspects.
ConclusionsThe conclusions that may be drawn about TO management based on the above review are as follows:
- •
New consensus diagnostic tests truly reflecting disease activity are needed.
- •
In the early and mild stages of TO, only symptomatic measures and selenium supplements are indicated.
- •
Smoking cessation is essential for TO control.
- •
Thyroid function control is another very important aspect on which most specialists are in agreement.
- •
Corticosteroid therapy and RT are indicated, whether associated or not, when moderate to severe activity exists.
- •
Surgical decompression is indicated for optic neuropathy resistant to corticosteroid therapy and RT, or in the absence of activity, for orbital decompression.
- •
Reparative surgery may also be required for strabismus and disabling palpebral conditions. It may also be indicated for cosmetic purposes.
- •
A multidisciplinary approach is highly recommended because of the complexity of TO.
- •
Quality of life assessment should be included as a routine tool both in trials and in the clinical management of TO.
The authors state that they have no conflicts of interest.
We thank María Ángeles Torres Berruezo for her invaluable help in the preparation of the figures illustrating the immune process.
Please cite this article as: Vilar-González S, Lamas-Oliveira C, Fagúndez-Vargas MA, Núñez-Quintanilla AT, Pérez-Rozos A, Merayo-Lloves J, et al. Orbitopatía tiroidea, una visión global con atención especial al papel de la radioterapia. Endocrinol Nutr. 2015;62:188–199.