The vitamin D hormone system has significant skeletal and extra-skeletal effects. Vitamin D receptor occurs in different tissues, and several cells other than renal cells are able to locally produce active vitamin D, which is responsible for transcriptional control of hundreds of genes related to its pleiotropic effects. There is an increasing evidence that relates vitamin D to development and course of type 1 and 2 diabetes mellitus. Specifically, influence of vitamin D on the renin-angiotensin–aldosterone system, inflammatory response, and urinary albumin excretion could explain the relevant impact of vitamin D status on diabetic nephropathy. Selective vitamin D receptor activators are molecules that are able to reproduce agonistic or antagonistic effects of active vitamin D depending on the tissue or even on the cell type. Specifically, paricalcitol has a beneficial profile because of its potency to reduce parathyroid hormone, with lower effects on serum calcium or phosphate levels. Moreover, in patients with diabetes and renal disease, paricalcitol decreases microalbuminuria, hospitalization rates, and cardiovascular mortality. Therefore, these molecules represent an attractive new option to improve prognosis of renal disease in patients with diabetes.
El sistema hormonal de la vitamina D es importante no sólo por sus conocidos efectos esqueléticos, sino también por sus potenciales efectos sobre otros órganos y sistemas cada vez mejor conocidos. El receptor de vitamina D está presente en gran cantidad de tejidos y células del organismo que, además, son capaces de producir la forma activa de vitamina D responsable de la activación de hasta 200 genes que pueden explicar los efectos pleiotrópicos de esta hormona. Existe una evidencia creciente que relaciona la deficiencia de vitamina D y la predisposición a desarrollar diabetes mellitus tipo 1 y tipo 2, así como la evolución de ambas enfermedades. En particular, la influencia de la vitamina D en el desarrollo de la nefropatía diabética es relevante por su interacción con el sistema renina-angiotensina-aldosterona, el proceso inflamatorio y la albuminuria. Los activadores selectivos del receptor de vitamina D son moléculas capaces de producir efectos agonistas o antagonistas sobre diferentes tejidos. En concreto, el paricalcitol muestra un perfil muy favorable por su potencia para reducir la hormona paratiroidea con menores efectos sobre las concentraciones de concentraciones de calcio y fósforo. Además, en pacientes diabéticos con enfermedad renal, este fármaco ha demostrado efectos beneficiosos sobre la reducción de albuminuria, la tasa de hospitalizaciones y la mortalidad cardiovascular. Por lo tanto, la posibilidad de utilizar una nueva herramienta para mejorar el pronóstico de los pacientes diabéticos con enfermedad renal en diferentes estadios es un reto que debe ser afrontado.
Vitamin D is a unique hormone because it may be formed in the skin from sunlight exposure. The terminology related to the biochemistry of vitamin D is somewhat complex. Vitamin D has two forms and several metabolites. The two forms are vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol). Vitamin D3 is produced in the skin in response to ultraviolet B radiation, or may be obtained from some foods (e.g. fatty fish, egg yolk, and liver) or nutritional supplements. Few natural foods contain significant amounts of vitamin D, and the main dietary sources are therefore these foods or supplements. Vitamin D2 occurs in some of the dietary vegetables and is commercially produced by yeast irradiation. Both vitamin D2 and D3 are used for the fortification of foods and the preparation of supplements.1
Vitamin D from skin or food is biologically inactive and requires initial hydroxylation in the liver by the enzyme 25-hydroxylase to form 25-hydroxyvitamin D (25OHD or calcidiol). Some of the studies have suggested that vitamin D2 may be metabolized more rapidly than vitamin D3, but with a regular intake both forms may be considered bioequivalent.2 However, 25OHD requires subsequent hydroxylation in the kidneys by the enzyme 1α-hydroxylase (CYP27B1) to a more biologically active form, 1,25 dihydroxyvitamin D [1,25(OH)2D or calcitriol]. Although 25OHD has biological activity, this is significantly lower (approximately 200 times) as compared to that of calcitriol. 1,25(OH)2D has the characteristics of a hormone, and vitamin D is therefore a prohormone rather than a vitamin. The structure of 1,25(OH)2D is similar to that of other steroid hormones. Like other hormones, 1,25(OH)2D circulates in concentrations in the picogram range which are 1.000-fold lower than those of its precursor, 25OHD.
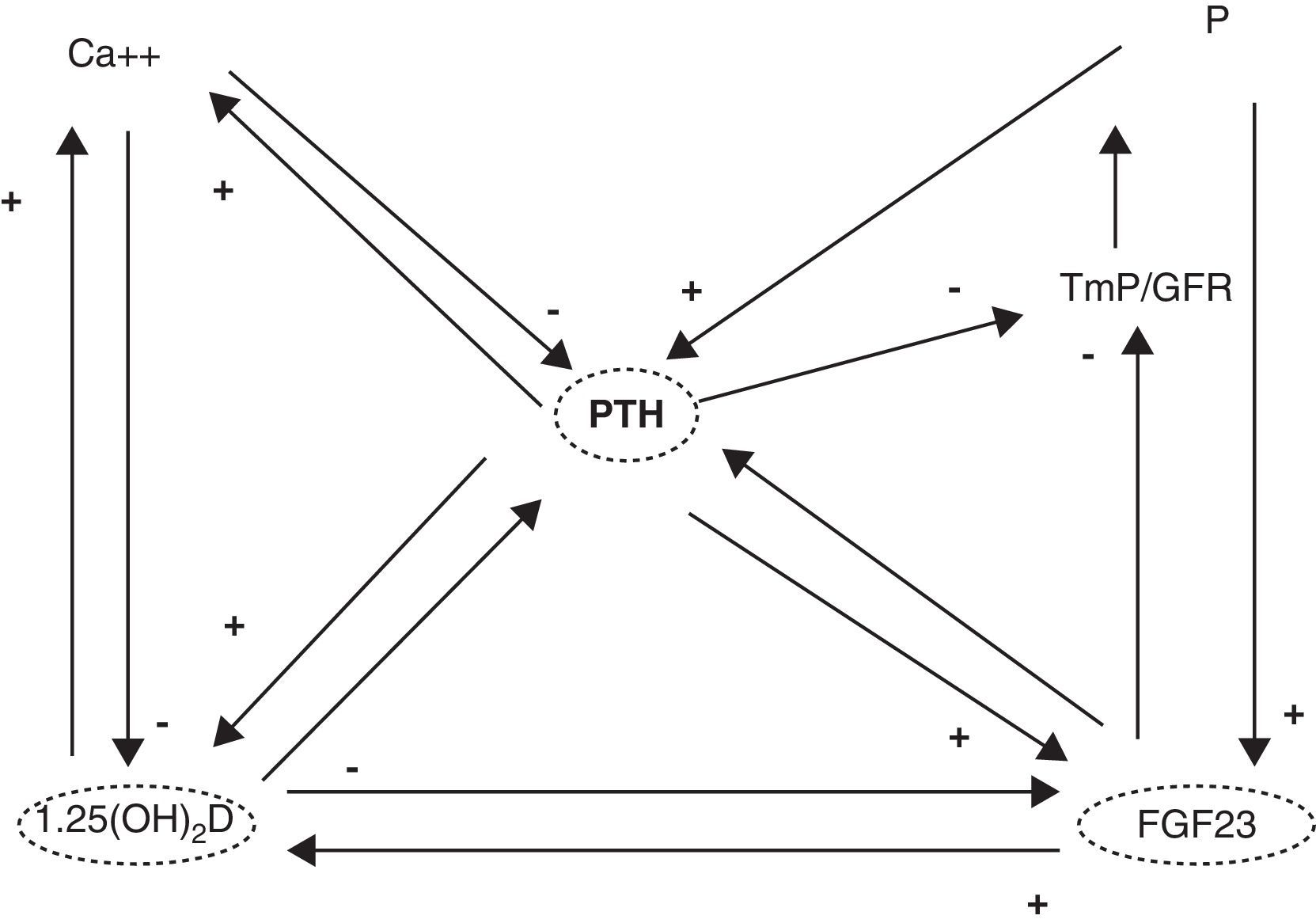
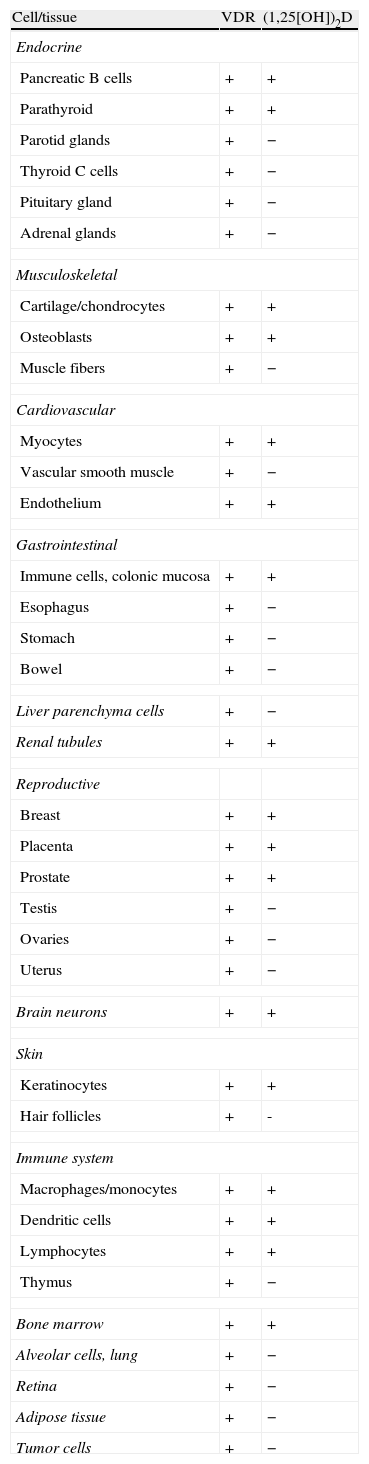
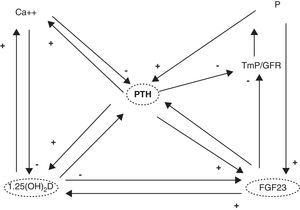
1,25(OH)2D acts upon its nuclear receptors, located in the small bowel, bone, kidneys, and other tissues.3 In the small bowel, 1,25(OH)2D stimulates the intestinal absorption of calcium and phosphorus. In bone, it interacts with bone cells, osteoblasts and osteoclasts, to mobilize calcium and other minerals. In the kidney, 1,25(OH)2D stimulates calcium reabsorption from the glomerular filtrate. Concentrations of this hormone are tightly regulated. Thus, parathyroid hormone (PTH) and low calcium and phosphorus levels increase its synthesis, while fibroblast growth factor-23 (FGF23), produced by osteocytes, decrease synthesis4 (Fig. 1). It should be noted that the vitamin D receptor (VDR) occurs in a large number of tissues and cells (Table 1). Therefore, 1,25(OH)2D has a broad spectrum of biological actions including the inhibition of cell proliferation, the induction of advanced differentiation, the inhibition of angiogenesis, the stimulation of insulin production, and the inhibition of renin production, amongst others.5 Moreover, several tissues and cells have 1α-hydroxylase activity. As a result, local production of 1,25(OH)2D is responsible for the regulation of up to 200 genes, which may explain the beneficial pleiotropic effects related to this hormone.6 It may therefore be stated that the vitamin D hormone system exerts its functions through two mechanisms: an endocrine mechanism (regulated by calcium, phosphorus, PTH, and FGF23) and a paracrine/autocrine mechanism (dependent on the vitamin D substrate, 23OHD and not regulated by factors regulating the endocrine mechanism). The purpose of the first mechanism is to achieve the homeostasis of mineral metabolism, and the second is to achieve all pleiotropic effects of the vitamin.
Cells and tissues producing calcitriol (1,25[OH])2D and/or having vitamin D receptors (VDR).
| Cell/tissue | VDR | (1,25[OH])2D |
| Endocrine | ||
| Pancreatic B cells | + | + |
| Parathyroid | + | + |
| Parotid glands | + | − |
| Thyroid C cells | + | − |
| Pituitary gland | + | − |
| Adrenal glands | + | − |
| Musculoskeletal | ||
| Cartilage/chondrocytes | + | + |
| Osteoblasts | + | + |
| Muscle fibers | + | − |
| Cardiovascular | ||
| Myocytes | + | + |
| Vascular smooth muscle | + | − |
| Endothelium | + | + |
| Gastrointestinal | ||
| Immune cells, colonic mucosa | + | + |
| Esophagus | + | − |
| Stomach | + | − |
| Bowel | + | − |
| Liver parenchyma cells | + | − |
| Renal tubules | + | + |
| Reproductive | ||
| Breast | + | + |
| Placenta | + | + |
| Prostate | + | + |
| Testis | + | − |
| Ovaries | + | − |
| Uterus | + | − |
| Brain neurons | + | + |
| Skin | ||
| Keratinocytes | + | + |
| Hair follicles | + | - |
| Immune system | ||
| Macrophages/monocytes | + | + |
| Dendritic cells | + | + |
| Lymphocytes | + | + |
| Thymus | + | − |
| Bone marrow | + | + |
| Alveolar cells, lung | + | − |
| Retina | + | − |
| Adipose tissue | + | − |
| Tumor cells | + | − |
Diabetes mellitus is a worldwide pandemic and is associated with microvascular and macrovascular complications and excess mortality. A recent report from the International Diabetes Federation states that the number of people with diabetes worldwide is coming close to 285 million, and will be 435 million in 2030.7 On the other hand, vitamin D deficiency or insufficiency is also a worldwide problem whose consequences go beyond its traditionally known effect on bone metabolism.8 Interest in the non-skeletal effects of this hormone system has increased because of the discovery of vitamin D receptors in various tissues such as pancreatic islet cells, immune system cells, macrophages, and vascular endothelium, amongst others. Through its paracrine effects, 1,25(OH)2D regulates the expression of different genes in these tissues.
There is an increasing evidence that relates vitamin D deficiency to the predisposition to develop type 1 and 2 diabetes mellitus, as well as to the progression of both conditions. The NHANES 2003–2006 study conducted on the US population surveyed 9773 adults of 18 years or more and showed an association between vitamin D levels, glucose homeostasis, and the progression of diabetes.9 Thus, an inverse relationship was shown between glycosylated hemoglobin (HbA1c) values and 25ODH levels in people aged 35–74 years with no known history of diabetes. Some of the authors therefore propose screening for vitamin D insufficiency in subjects with elevated HbA1c and vice versa.10 The underlying biological mechanisms are not well-known, but there are studies which have provided data that may explain this association. An example is the demonstration of 1,25(OH)2D receptors and 1α-hydroxylase enzyme activity in pancreatic β cells.11 Interventional studies with vitamin D supplements have reported a favorable effect upon glucose tolerance and insulin resistance in humans. In a recent meta-analysis, Pittas et al. have reviewed the impact of vitamin D and calcium on blood glucose control in patients with type 2 diabetes.12 Their results show that vitamin D insufficiency may make blood glucose control difficult and that supplementation with both nutrients may be required to optimize metabolic control. These data are consistent with those collected in studies conducted in animal models. The biological mechanisms suggested include the normalization of transmembrane calcium flow, required for insulin secretion, and increased insulin receptor expression.13 In addition to the relation of vitamin D to diabetes, various epidemiological studies have shown an association between vitamin D insufficiency and cardiovascular mortality.14 An increased risk of obesity, high blood pressure (HBP), and dyslipidemia appear to be the determinant factors. However, the available interventional studies have not consistently shown a favorable effect upon cardiovascular events.15
Vitamin D also interacts with the metabolic pathways involved in the occurrence and progression of the complications inherent in diabetes, particularly nephropathy. The most remarkable relations include those affecting the renin-angiotensin-aldosterone system (RAAS), the inflammatory process, and albuminuria.16 Vitamin D is a negative regulator of RAAS, as shown in animal models not expressing the vitamin D receptor.17 In a chronic renal disease model, a vitamin D analogue was able to decrease renal renin expression, renin receptor, angiotensinogen, and angiotensin type 1 receptor.18 Vitamin D also inhibits the tumor necrosis factor-α converting enzyme (TACE) that regulates angiotensin converting enzyme-2 (ACE2), the main enzyme metabolizing angiotensin II in the proximal tubule. Diabetes is associated with reduced ACE2 expression. TACE inhibition may therefore improve RAAS balance in the kidney and may have additional renal protective effects through TACE-dependent inhibition of other pathogenetic mediators.19
Vitamin D has several anti-inflammatory actions, including effects on prostaglandin synthesis, the inhibition of nuclear factor κβ, and innate immunity. Thus, some of the drug interventions have shown a favorable effect on the inflammatory profile.20 All these effects have been implicated in chronic renal disease of diabetic patients. In addition, vitamin D deficiency is associated with an increase in C-reactive protein (CRP) levels. Some of the studies with vitamin D analogues have shown their ability to decrease CRP levels, in parallel to albuminuria, in patients with chronic renal disease.21
Diabetes is associated with an aberrant shedding of megalin and cubilin, the main endocytic transporters of albumin filtered in the proximal tubule.22 This recovery pathway is essential for 25OHD uptake and subsequent activation. Dysfunction or deficiency of the cubilin-megalin complex decreases vitamin D levels. Significantly, this interaction acts through both pathways, because vitamin D also inhibits megalin elimination.
Patients with diabetes and chronic renal disease have high rates of vitamin D deficiency. Urinary protein losses related to vitamin D, decreased renal formation of 1,25(OH)2D and reduced expression of the vitamin D receptor (VDR) are characteristics of these patients.23 Vitamin D supplementation appears therefore to be justified in these cases. The availability of selective vitamin D receptor activators with less risk of hypercalcemia or hyperphosphatemia is particularly attractive in the management of these patients.24
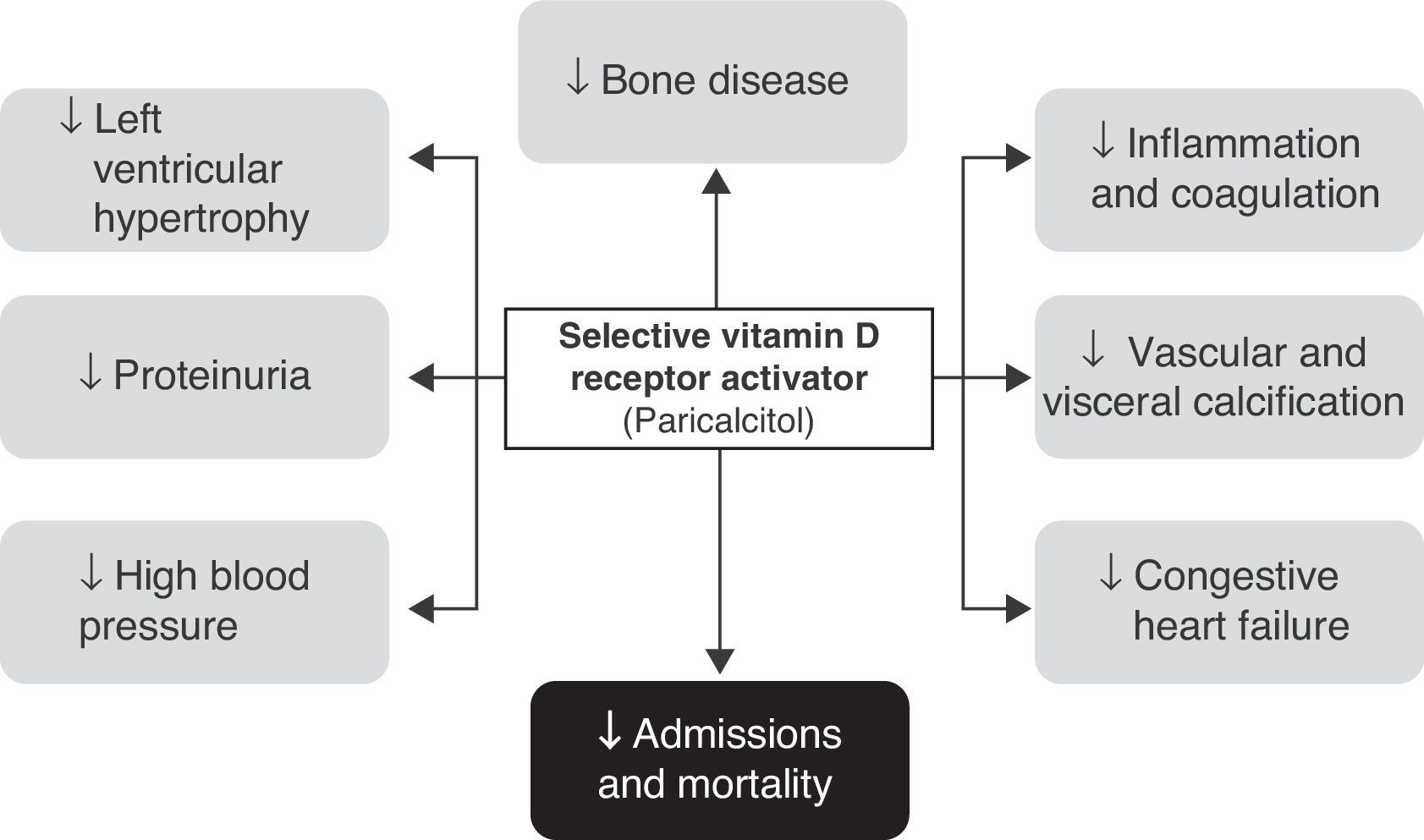
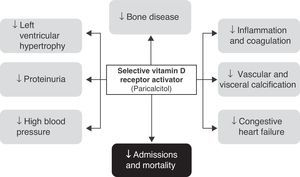
Selective vitamin D receptor activatorsThe therapeutic use of active D hormone (calcitriol) in chronic renal disease is intended to increase intestinal calcium absorption, thus protecting bone from the development of osteomalacia and inhibiting PTH secretion and parathyroid hyperplasia. In addition, the wide tissue distribution of VDR (kidney, pancreas, myocardium etc.) may provide additional benefits, although the development of hypercalcemia or hyperphosphatemia restricts or complicates its use. It is currently thought that a significant part of the complications occurring in patients with chronic renal disease (especially those related to hypertension, left ventricular hypertrophy, vascular calcifications, and proteinuria, in addition to purely bone complications such as secondary hyperparathyroidism) are related to deficient VDR activity.25
As discussed above, vitamin D is actually a hormonal secosteroid with receptors distributed over many tissues and related to sex hormones. In fact, the concept of selective steroid hormone modulator arose from the study of different substances with estrogenic activity in some tissues and anti-estrogenic activity in others during the 1980s.26,27
The complex mixed agonist/antagonist effects of those compounds led to their current name of selective vitamin D receptor modulators or activators. At the molecular level, we are only just starting to discover how a specific selective modulator or activator acts as an agonist on the VDR of a particular tissue or cell and as an antagonist on another. It appears that conformational changes (in the three-dimensional structure and electric charges of the VDR after binding to a specific ligand, in addition to homodimerization or heterodimerization with RXR) determine which proteins from the cell nucleus (containing both genetic material with response elements to vitamin D and VDR itself) will bind to the activator increasing (co-activators) or inhibiting (co-repressors) expression of those genes. In addition to the genomic effects modifying protein expression of multiple genes, these substances may have non-genomic effects.28
Different vitamin D analogues29 have been developed to treat secondary hyperparathyroidism in patients with chronic renal disease, but with a low risk of hypercalcemia and hyperphosphatemia. Their effects depend on both their pharmacokinetic differences (binding to transport proteins, rate of uptake and metabolism and catabolism by target cells) and pharmacodynamic differences: interaction sites with the VDR ligand cavity, conformational differences of the receptor-hormone complex, interactions with co-factors of the RXR/VDR/co-activators complex, co-repressors, or proteosomes accounting for differences in actions in each tissue and/or cell.28
Among these analogues, paricalcitol [19-nor-1,25-(OH)2-vitamin D2] appears to have the best profile, because it has a lower risk of inducing adverse effects on serum calcium and phosphorus levels and a greater potency for decreasing PTH levels,25,29 and has been shown to have additional benefits in diabetic patients such as the reduction of albuminuria and hospitalizations and mortality in patients with chronic renal disease.30,31 The several clinical studies carried out with paricalcitol have allowed us to understand more clearly the effects of selective VDR activators and to assess its potential applications beyond the control of PTH levels in subjects with diabetes.
Effects of paricalcitol in diabetic patients with renal diseaseIt should first be noted that many of the available studies were not specifically focused on diabetic patients with renal disease. In fact, most of the available evidence comes from studies having as inclusion criterion the presence of moderate to end-stage chronic renal disease (on regular dialysis). However, in studies where this information is available, virtually half the patients recruited had diabetes, and post hoc analyses did not show a lower benefit in this population (Fig. 2).
Effect on PTHParicalcitol was developed to treat hyperparathyroidism secondary to chronic renal failure due to the deficient production of active D hormone without the adverse effects of hypercalcemia and hyperphosphatemia that may be associated with treatment with active vitamin D. Paricalcitol was shown to be effective and safe in this setting in three 12-week controlled clinical trials (some with an extension lasting up to one year) in recently reviewed patients on regular hemodialysis.31 Overall, these studies have demonstrated a 60% PTH reduction with a low risk of hypercalcemia and hyperphosphatemia.
Its efficacy has also been compared to that of calcitriol in 263 patients on regular dialysis32 in whom reduction of PTH levels by at least 50% was confirmed. Paricalcitol showed a faster action than calcitriol and induced significantly less episodes of hypercalcemia or increase in calcium-phosphorus product. This efficacy of paricalcitol for the sustained reduction of PTH levels was also confirmed in patients who were in earlier stages (3–4) of renal failure.33
The mechanism of paricalcitol which decreases its calcemic effect is related to a lower stimulation of the enterocyte proteins responsible for the absorption of dietary calcium (calbindins and others) as compared to calcitrol,34,35 which results in the selective activation of VDR.
Effects on proteinuriaThe hypothesis of the potential effects of paricalcitol on albuminuria is derived from the known pleiotropic effects of vitamin D and, specifically, its previously discussed effects as an immunomodulator and in decreasing RAAS activity. Excess protein loss is undoubtedly a clear risk factor for the progression of renal disease and cardiovascular risk in patients with both chronic renal failure36 and diabetes.37 Thus, the activation of VDR in the tubular cell by calcitriol increases megalin expression and, therefore, the reabsorption of 25OHD and albumin from the tubular lumen. A joint analysis of proteinuria, assessed using urinary dipsticks as a safety analysis in the three cohorts showing the efficacy of paricalcitol in decreasing PTH, provided the first positive results. Paricalcitol was shown to improve proteinuria in 29 of 57 patients (51%), as compared to 15 of the 61 patients (25%) given placebo (p=0.004; odds ratio [OR] 3.2). These results did not change after adjusting for age, sex, race, HBP, or the use of RAAS blockers. An additional and more interesting finding for us as endocrinologists was that patients with diabetes experienced the same benefit as the rest of the cohort.38 These data were supported by a pilot clinical study of 24 patients that reported an almost 50% reduction in formally measured proteinuria19, and by a subsequent study of 61 patients with different grades of renal failure (glomerular filtration rate, 15–90mL/min/1.73m2) and proteinuria (>400mg/24h) in whom six months of treatment with paricalcitol decreased proteinuria by 17.6%, as compared to the 2.9% reduction seen with placebo (p<0.04).39
However, the most significant data about albuminuria reduction come from a study of 300 subjects with type 2 diabetes and albuminuria (approximately 1g/24h) despite treatment with RAAS inhibitors who were treated with placebo or paricalcitol 1 or 2μg. Overall, the patients treated with paricalcitol showed a 15% reduction in albuminuria as compared to the placebo group (10% and 20% versus placebo for the 1 and 2μg doses respectively), with no difference in adverse effects.40 These data from diabetic patients provide clear evidence for a new approach to the prevention of albuminuria in the early stages of renal failure (3 or higher) and to the reduction of the high residual renal risk in this population.
It should finally be noted that an elevation of serum creatinine and creatinine clearance has been seen in patients treated with paricalcitol, although more specific studies show that the clearance of creatinine and iotalamate does not change and that albuminuria is significantly reduced with paricalcitol. It is therefore assumed that paricalcitol increases creatinine generation and serum levels in the short term, but does not affect the glomerular filtration rate.41
Cardiovascular effectsThe disease itself and its cardiovascular complications are the main causes of mortality in subjects with both diabetes37 and chronic renal disease.42 Traditional risk factors such as smoking, age, or high blood pressure do not completely explain this high morbidity and mortality, and the best results are achieved with aggressive multifactorial treatment.37
Effects on the renin–angiotensin–aldosterone systemThe renin–angiotensin–aldosterone system (RAAS), a regulator of extracellular volume homeostasis that contributes towards stabilizing blood pressure, is clearly implicated in the occurrence of complications associated with diabetes and renal disease. RAAS inhibition has been shown to have significant benefits in the prevention and treatment of chronic renal disease of diabetic or other etiologies and a favorable impact on the very high cardiovascular risk of these patients.37 Recent experiences show that active vitamin D and VDR activators have a suppressing effect on RAAS by decreasing renin gene expression.43 The anti-inflammatory or anti-fibrosing effects of selective vitamin D activators are additional positive actions that make these molecules attractive as a potential treatment for diabetic patients with kidney damage. VDRs also exist in the cardiovascular system, where they modulate the expression of a high number of genes.44,45
In experimental models of almost total nephrectomy, paricalcitol was shown to decrease the mRNA expression of angiotensinogen, renin receptor, and vascular endothelial growth factor by 30%-50%. Such mRNA reductions were associated with the expected decreases in protein concentrations (renin, renin receptor, angiotensin type 1 receptor, and vascular endothelial growth factor) and to an improvement, suggested by the authors to be related to RAAS blockade, in tissue damage (tubulointerstitial) associated with the model of chronic renal failure, hypertension, proteinuria, and kidney function impairment.17
In fact, paricalcitol co-administered with losartan in diabetic models has been shown to prevent the relative loss of efficacy of RAAS inhibitors caused by a compensatory increase in renin. In these animal models of type 1 and 2 diabetes, co-administration prevents the kidney damage caused by diabetes (progressive proteinuria and glomerulosclerosis), restoring the glomerular filtration barrier by suppressing the expression of fibronectin, transforming growth factor β, and monocyte chemoattractant protein-1 and restoring the slit diaphragm proteins nephrin, Neph-1, Zo-1, and α-actinin-4. These benefits are associated with the blockade of intrarenal renin and the accumulation of angiotensin II induced by hyperglycemia and losartan.46,47
Effects on left ventricular hypertrophyThe relationship between renal failure and the development of left ventricular hypertrophy (LVH) has been well-known for years.48 A prevalence of concentric and eccentric LVH up to 87% has been reported in predialysis patients. LVH is also related to PTH levels, and improves after parathyroidectomy in patients with hyperparathyroidism secondary to advanced renal failure.49
Paricalcitol reduced LVH in animal models of renal failure (uremic rats due to subtotal nephrectomy).50 This improvement in LVH was associated with the upregulation of VDR and to reductions in proliferating cell nuclear antigen (PCNA, a nuclear protein expressed in the early G1 and S phases of the cell cycle, a co-factor of DNA polymerase) and myocardial oxidative stress.51
Findings in Dahl salt-sensitive rats, which developed HBP on a salt-rich diet are even more interesting. In this context, paricalcitol, as compared to placebo, resulted in lower heart and lung weights, lower left ventricular mass, posterior cardiac wall thickness, and end-diastolic pressure without affecting blood pressure, but significantly decreased concentrations of brain natriuretic peptide (BNP), atrial natriuretic peptide and renin mRNAs.52 Some other data in patients also illustrate this role. A retrospective study of 38 subjects with renal failure treated or not with paricalcitol for 12 months showed an improved left ventricular diastolic function and a reduction in posterior wall (11%) and septal (15%) thickness in echocardiography.53 However, a recently reported clinical trial of paricalcitol in patients with chronic renal disease showed no favorable effects on LVH.54
Effects on vascular calcificationsVascular calcifications are clearly associated with a greater increase in cardiovascular risk, particularly not only in patients with advanced chronic renal disease,55,56 but also in the general population or in diabetic patients in whom they have been shown to have a predictive capacity after adjustment for the presence of diabetes.57,58
In experimental models, relevant doses of paricalcitol are associated (and correlated) with a lower calcium deposit and a better therapeutic outcome, while very high doses may induce vascular calcification. Thus, paricalcitol doses equivalent to the therapeutic doses decrease aortic calcification and, at molecular level, gene expression in the aortic cells of osterix, Msx2, Cbfa1/Runx-2, and osteocalcin, osteoblastic and osteogenic markers, as well as progression markers of vascular calcification, although not all VDR activators are as effective or safe at doses equivalent to those used in the clinical setting.59,60 Thus, this reduction of calcification maintains the normal values of pulse wave velocity, a measure of vascular stiffness.61,62
Effects on coagulationDiabetes is associated with hyperactivity of the systems controlling coagulation.63 Both the plasminogen activator inhibitor (PAI-1), thrombospondin-1 (TBS1), and thrombomodulin (TM) have been implicated in the development of atherothrombosis.
In cultures of aortic smooth muscle cells (SMCs), paricalcitol and calcitriol dose-dependently downregulate both PAI-1 (mRNA and protein) and TBS1, and upregulate both TM mRNA and protein (a protein with antiangiogenic properties), which appears to be the target gene of vitamin D and paricalcitol.64 While VDR and the enzymes involved in its metabolism have not been detected in endothelial cells, the significance of these findings in patients with diabetes is clear.
Other effects: Effects on inflammation, immunomodulatory role, insulin production, and tumorsThe role of chronic inflammation in the development of organ damage in multiple diseases, such as kidney damage in chronic renal disease, pancreas damage in diabetes, or vascular tree damage in cardiovascular disease, is increasingly being recognized. Paricalcitol retains the immunomodulatory effects of calcitriol by altering the differentiation of immature dendritic cells from monocytes, blocking the production of interleukin-12 and, thus, of Treg cells.65 In kidneys damaged by a renal flow obstruction model, a reduction is seen in inflammatory infiltration. This is associated with a decreased expression of RANTES chemokines and tumor necrosis factor α and is related to the inhibition of the NF-κB pathway.66 Epidemiological studies suggest that vitamin D deficiency may have an etiological role in several human cancers.67 Since this immunomodulatory role occurs with calcitriol, it results in antiproliferative effects on the prostate and pancreatic cancer lines,68,69 together with positive data on the modulation of insulin secretion by calcitriol and possibly paricalcitol,70 and renders the use of paricalcitol in currently ongoing studies of diabetes prevention and/or early treatment highly attractive (http://clinicaltrials.gov/ct2/show/NCT01003275).
Finally, there have been reports of the antiproliferative effects of paricalcitol on cell cultures and experimental models of myeloid leukemia and colonic cancer mediated by its action on VDR, leading to clinical trials in these diseases being proposed.71
Effects on hospitalizations and mortalityA retrospective study had reviewed the clinical histories of 67,399 patients treated with calcitriol or paricalcitol alone. Patients were evaluated over a two-year period using the following endpoints: kidney transplantation, death, a switch to another vitamin D formulation, or patient referral to another hospital. Patients treated with paricalcitol showed greater survival as compared to those given calcitriol (mortality rate, 0.180/patient/year versus 0.223 for calcitriol, p<0.001). The difference was significant at 12 months and increased with follow-up time. Mortality reduction after adjustment was 16%. Calcitriol was not superior in any of the 42 strata analyzed, while paricalcitol was superior in 28 strata.24 Other studies also showed improved survival and a 14% reduction in hospitalizations (0.64 less admissions per year) in patients treated with paricalcitol.72–74 Increased survival appears to be related to a reduction of cardiovascular morbidity and mortality.75 Prospective studies confirming these data are obviously needed, although the favorable impact of paricalcitol has been consistent to date. It should also be noted that diabetic patients accounted for approximately 50% (45–48%) of the patients enrolled in these studies, and that the benefits of paricalcitol in them were indistinguishable from those seen in the whole group.
Conclusion and perspectivesThere is ample evidence which shows the relationship between the hormonal, and also paracrine and autocrine, vitamin D system and the course and prognosis of diabetic patients with kidney disease. The development of selective vitamin D receptor activators with an improved safety profile, such as paricalcitol, provides a new tool for the management of these patients, who currently have unacceptable morbidity and mortality rates. The first step should be the correction of vitamin D deficiency, the underlying mechanism of pleiotropic actions. The conduct of new studies to precisely identify the benefits of intervention with this type of drug in the different stages of kidney disease in diabetic patients is also warranted.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Jódar-Gimeno E, Muñoz-Torres M. Sistema hormonal D y diabetes mellitus: lecciones de los activadores selectivos del receptor de vitamina D. Activadores selectivos del receptor de vitamina d y diabetes mellitus. Endocrinol Nutr. 2013;60:87-95.