Fecal immunochemical test (FIT) is a screening tool for detecting neoplastic lesions in asymptomatic patients. Colonoscopy is indicated in levels of FIT >100ngHb/ml.
AimCompare the frequency of advanced adenomas (AA) in colonoscopy between asymptomatic subjects with FIT+ vs. symptomatic patients. Evaluate the level of bleeding of AA.
Material and methodologyCross-sectional, observational study of 2829 consecutive patientes referred for colonoscopy. We collected clinic-demographic information, AA (size, topography, and histology) and FIT quantification. We included 2829 subjects who underwent colonoscopy.
Statisticsχ2, Student's t-tests (0.05), one-way ANOVA and ANCOVA were performed for adjusted contrasts among lesions, gender, positive left colonic findings and age.
ResultsGlobal rate of AA was 7.2%. Detection of AA was higher in average-risk asymptomatic subjects than in symptomatic (8.9 vs. 4.35%); no differences in age, sex or site of lesion was find. Group 1: (n=902). 105 AA (alone: 80.2%; ≤9mm: 27.2%; 10–15mm: 62.9% and ≥16mm: 9.9%) were detected in 81 patients (females: 38.2%; mean age: 64.1±8.4 years). Group 2: (n=1927). AA 105 in 84 (5.1%) in symptomatic patients.
Quantitative FIT showed a correlation between AA size and bleeding level. For each mm that increased the size of the AA, the bleeding level increased 35±9ngHb with a stop limit of 15mm.
ConclusionsDetection was higher in average-risk asymptomatic subjects with FIT+ than in symptomatic patients. Quantitative FIT showed a correlation between adenoma size and bleeding level.
La Prueba Inmunoquímica (FIT) es una herramienta de tamizaje para detectar sangre oculta en heces en sujetos asintomáticos. Pacientes con prueba positiva (≥100ngHb/ml) son referidos para colonoscopía.
ObjetivoComparar la frecuencia de Adenomas Avanzados (AA) detectados en colonoscopía entre sujetos asintomáticos FIT+ vs. pacientes sintomáticos. Determinar el nivel de sangrado de los AA.
Materiales y métodosEstudio observacional con análisis transversal en 2,829 pacientes consecutivos enviados a colonoscopía. Se tomaron datos clínico-demográficos, edad, sexo, presencia de adenoma avanzado (tamaño, topografía, e histología) y valor del FIT. Incluimos sujetos con colonoscopia (902 FIT+ y 1,927 sujetos sintomáticos).
EstadísticaPruebas X2, t de student (0.05), ANOVA y ANCOVA para ajustar contraste entre lesiones, sexo, hallazgos en colon izquierdo y edad.
ResultadosFrecuencia global de AA, 7.2%. Fue mayor en asintomáticos (riesgo promedio) (8.9 vs. 4.35%) que en sintomáticos, sin diferencias en edad, sexo o sitio de la lesión. Grupo 1: (n=902); 105 AA (únicos: 80.2%: ≤9mm, 27.2%: 10–15mm: 62.9% y ≥16mm: 9.9%) en 81 sujetos (mujeres: 38.2%; edad: 64.1 ± 8.4 años).
Grupo 2(n=1,927), AA 105 en 84 (5.1%) pacientes sintomáticos.
La cuantificación del FIT mostró relación entre el tamaño del AA y el nivel de microsangrado. Por cada mm que aumenta el tamaño del AA, el microsangrado aumenta 35 ± 9ngHb, pero el valor del FIT deja de incrementarse en los 15mm.
ConclusionesLa detección de AA fue mayor en pacientes asintomáticos con riesgo promedio y FIT+. La prueba mostró relación proporcional entre el tamaño del AA y la magnitud de la microhemorragia.
Colorectal cancer (CRC) mainly affects the economically active population and is the most frequent digestive neoplasms. One element of great importance in prevention and timely detection programs is the increase in the incidence of CRC in subjects aged between 45 and 50 years.1,2 The primary objective of the screening was to detect CRC in asymptomatic subjects.1 The precancerous phase (adenoma) is asymptomatic; presents in up to 90% of cases and is due to a non-linear accumulation, scaled, number of genetic alterations and chromosomal alterations, which occur during the time course of approximately 7–10 years.3
Advanced adenoma (AA) is defined as an adenoma with one of the following items: size >10mm, villous component >25% and/or High-grade dysplasia (HGD)4,5; these have a greater risk of malignization (50%); thus, they must be resected. The risk increases in synchronous AA, mainly in the elderly.4 After resection, the patient should begin a colonoscopic surveillance program to detect metachronous adenomas prior to their malignant transformation.6
Surveillance programs (the National Polyp Study) recommend colonoscopy 3 years after the resection of an AA, because there is a 3.3% rate of detection of new polyps.7,8 Adenomas can be detected in the colonoscopy through screening or by reference symptoms (digestive bleeding). A characteristic of early CRC and most of the Advanced Adenoma is the occult bleed; tests that detected this bleeding allowed the early diagnosis of these lesions.9
In the Early Prevention and Detection of CRC Program at the Digestive Cancer Center (DCC), we use immunochemical test to detect occult blood in feces (FIT – OC Hemodia (Eiken Chemical Co., Tokyo, Japan)) as a tool for screening. A positive test (≥100ngHb/ml) suggests a high probability of occult bleeding or microbleeding associated with benign or malignant lesions; thus, the patient should be referred for colonoscopy (screening).10–15
The objective of the study was to compare the colonoscopic detection rate of AA in asymptomatic subjects (average risk) with a positive FIT test vs. symptomatic subjects. A secondary objective was to evaluate the usefulness of quantification FIT in the correlation between adenoma size and bleeding.
Materials and methodsWe conducted an observational, comparative study with transversal analysis of a series of consecutive cases of asymptomatic subjects with average risk (FIT-positive) and patients with colonic symptoms submitted to program colonoscopy at the CDC of the National Cancer Institute of Montevideo, Uruguay. In the 2829 colonoscopies performed, 205 (7.2%) lesions were found with endoscopic and/or histological criteria of advanced adenoma:
Group 1: Asymptomatic subjects with average risk with positive FIT (n=902), and Group 2: symptomatic patients (n=1927).
Data were captured in a Lotus Approach version 9.7 for Windows database and included age, gender, topography, size, histopathological diagnosis and quantification of FIT. We also considered patients with two or more advanced adenomas; in these cases, we analyzed the largest-sized lesion or that localized nearest the cecum.
Operational description of the variablesSubjects at average risk for CRC: Asymptomatic subjects aged >50 years, without antecedents of CRC of adenomas.2–4
Advanced adenomas: Adenomas >10mm or villous component >25% or high-grade dysplasia (HGD).3,4
Fecal immunochemical test: A screening tool for investigating occult blood in fecal matter by means of an immunochemical test (Fecal Immunochemical Test – OC-Eiken [Eiken Chemical Co., Eiken, Japan]) (FIT), which is a human antihemoglobin antibody based on a reaction of latex agglutination that possesses the advantages of high sensitivity, which does not activate with animal hemoglobin nor with peroxidase of plant origin; thus, it does not require a special diet and is easy to apply.8,9 Detect bleeding of the distal ileum, colon and rectum and do not have interference with the intake of NSAIDs. The test has high specificity (96–98%) and sensitivity (91–95%), and is a qualitative and quantitative method because automatic processing allows determination of the level of hemoglobin contained in the stool.10,16 The detection range is from 0 to 2000ngHb/ml of buffer, which allows fixing the cut-off point for a positive result; the cut-off point at our Center and according to the recommendation of the manufacturers was set at 100ngHb/ml.9,13
Symptomatic subjects: Patients with different colonic symptoms, submitted for the first colonoscopy.
Statistical analysis: We utilized the χ2 and the Fisher exact tests for categorical variables and the Student's t-test with a significance level of 0.05 to evaluate differences for independent samples. We employed one-way Analysis of variance (ANOVA) and analysis of covariance (ANCOVA) to adjust the contrast among the lesions in terms of gender, positive colonic findings, and age. A hyperbolic function was calculated for two parameters. We utilized SPSS version 17.0 for Windows and Epi Info version 3.3.2.0 statistical software.
ResultsGroup 1: Eighty one of 902 (8.9%) asymptomatic subjects had a FIT+ (31 women; average age, 64.1±8.4 years) had 105 AA (11.5%), 65 had one AA (80.2%) and 16 had two or more AA (19.8%). Size was >10mm in 73.8% of cases (22 AA, ≤9mm; 51 AA, 10–15mm, and 8 AA, ≥16mm). No significant differences were observed were between age and AA size (Table 1).
Group 2: Eighty four of 1927 (4.35%) patients with symptoms had 100 AA (5.1%).
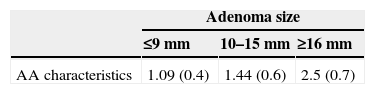
Considering the presence of one of more characteristics of AA (HGD/≥25% villous component/size ≥10mm), it was observed that those smaller in size presented only one characteristic, and those of a larger size, >2 characteristics (Table 2).
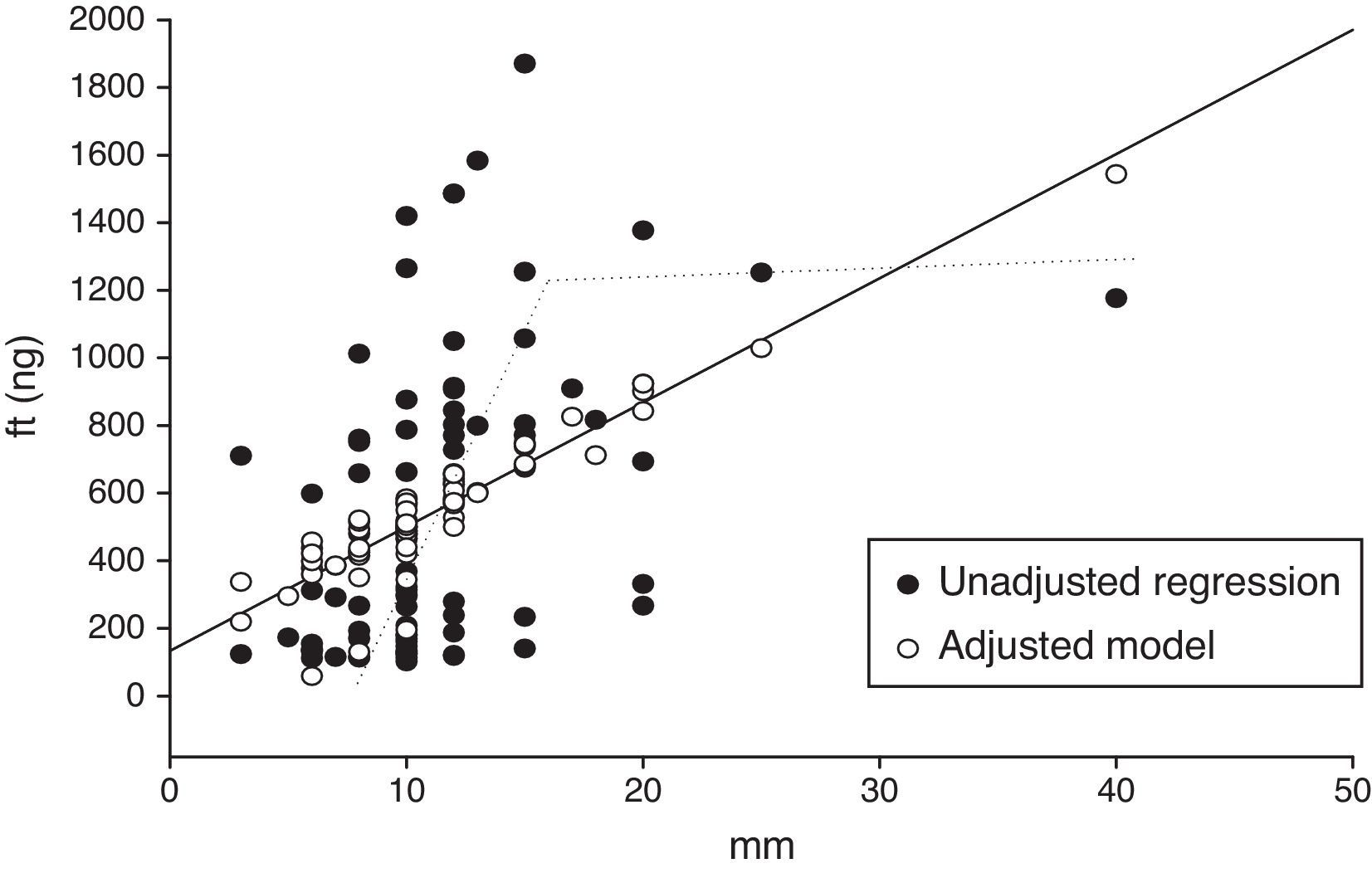
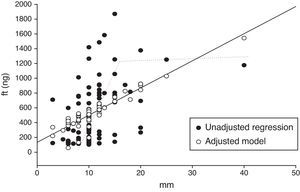
Quantification of the bleeding using FIT presented a directly proportional relationship with the size of the adenoma (Table 3) with a 15mm inflection point, at which the values stopped increasing, becoming a nearly flat curve of between 1100 and 1900ngHb (Fig. 1).
We used covariate analysis to determine the association between lesion size and microbleeding intensity, adjusted for gender, lesion site and age. The parameters show that for each mm of increase in size, the FIT increased the hemoglobin value in 35±9ng (p<0.001). No significant differences were observed in analysis by age, gender, or lesion site.
DiscussionIn our series, the global frequency of advanced adenomas detected by colonoscopy was 7.2%. The detection rate was higher in asymptomatic subjects with a positive fecal immunochemical test (FIT) than in patients with symptoms referred for colonoscopy (8.9 vs. 4.3%). Quantification of the bleeding showed a directly proportional relationship with the adenoma size and a 15mm AA size inflection point, at which the values stopped increasing, becoming a nearly flat curve between 1100 and 1900ngHb.
Negron et al.4 reported a global frequency of 9.6% of AA. When these authors analyzed the indication for colonoscopy (symptomatic or FIT-positive), they observed that while the number of AA detected for each group was similar (100/105 AA), frequency in symptomatic patients (5.1%) was less than with a FIT+ (11.6%). Our study reported a global frequency of 7.2% of advanced adenomas, with 8.9% with FIT positive and 4.3% in symptomatic patients; numbers are very similar to those of the previously mentioned authors.
The frequency of synchronous adenomas was of 30–50% without discriminating whether the adenomas were advanced or not.8
In our series, nearly 20% had a synchronous AA. The frequency of metachronous AA and a recurrence was reported as 9.37% (range, 36–72 months)6 and 6.5% at 4 years in the postpolypectomy follow-up.17
Early detection programs for AA exert an impact on the reduction of the incidence of cancer, because due to its high risk of malignant transformation, endoscopic or surgical resection interrupts the Adenoma–Cancer sequence.15–18 Winaver19 suggested evaluating the usefulness of screening programs by means of AA detection rate, nutritional interventions and novel chemoprevention agents. In 2006, our work team reported a global diagnosis of AA (1.24%) in the 10,573 patients who participated in the Colorectal Cancer Prevention and Detection Program15 and a detection rate of 14.9% in patients with a positive FIT and colonoscopy.
Usually, the objective of screening in subjects with average risk is having a >95% specificity for CRC. This is obtained with a cut-off point of 100ngHb/ml of FIT.20
Rozen et al.21 informed ideal cut-off points (Receiver operating characteristics, [ROC]) for detecting CRC or AA and for AA alone using one, two or three tests. The greatest Area Under the Curve (AUC) was reached with two tests for detecting CRC (0.957; 95% Confidence interval [95% CI], 0.935–0.978; p=0.67); no differences were found with the AUC between the number of tests for CRC or AA and AA alone. The positivity rate among the three tests was not significantly different (Cochrane test).
The optimal cut-off point has an economic impact. If the cut-off point is determined at 50ngHb/ml, sensitivity will increase, specificity will be reduced, but additional colonoscopies will be necessary and many persons will have irrelevant findings (false positives). When the cut-off point is increased to 100ngHb, specificity will increase, with the need for fewer colonoscopies, but there will be the risk of overlooking malignant lesions.22 That is, an equilibrium between the sensitivity and specificity of the test will be required (ROC curve) to achieve greater cost-effectiveness.
While not yet demonstrated to date, a greater risk of malignization should exist in AA that present >1 of the endoscopic or histological characteristics that define AA. In this regard, we observed that nearly all adenomas of >16mm in size had a villous component and/or HGD. Another fact to highlight is that larger lesions size had higher bleeding. Lesions >16mm in diameter double the bleeding magnitude when compared with those <9mm in diameter; however, as Table 1 shows, all lesions >15mm had significant bleeding values and these values were similar, independently of the size. There is no evidence to suggests that magnitude of the bleeding correlates with the adenoma's topography.23
ConclusionsIn our series, the global frequency of advanced adenomas detected by colonoscopy was 7.2%. The high rate of detection justifies a deeper analysis of their characteristics. The detection rate was higher in asymptomatic subjects with a positive FIT than in symptomatic patients referred for colonoscopy (8.9 vs. 4.35%). Bibliography not reported the relationship between bleeding value and advanced adenoma size; in our study the FIT increased 35±9ngHb/ml for each mm of increase in size of the AA. Quantification of the hemorrhage showed a directly proportional relationship with adenoma size with a 15mm inflection point, at which values stop increasing, becoming a nearly flat curve between 1100 and 1900ng/Hb.
Ethical responsibilitiesProtection of people and animalsThe authors state that the procedures conformed to the ethical standards of human experimentation committee responsible and according to the World Medical Association and the Declaration of Helsinki.
Confidentiality of dataThe authors declare that they have followed the protocols of the workplace on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the informed consent of patients and/or subjects referred to in Article consent. This document is in the possession of the corresponding author.
Conflict of interestThe authors declare no conflict of interest.
FundingThe authors did not receive sponsorship to undertake this article.