Introduction
Non-variceal upper gastrointestinal bleeding (NVUGIB) is a life-threatening disorder accounting for more than 100 000 admissions per year with a cost of more than $2 billion annually in the United States.1 There are several Score Systems to support early discharge of patients with low-risk lesions on endoscopy.2-4 Although extremely useful, they cannot be completed without the endoscopic findings and, therefore, cannot be used before endoscopy. Also, the need for an immediate endoscopy cannot be determined with such score systems. Recently, a modified Blatchford Score Risk System5 (mBRS) which exclude the endoscopy findings, was reported with good results to identify patients with gastrointestinal bleeding with a low likelihood of having high risk stigmata ulcers (HRSU) and a low risk of adverse outcomes (rebleeding and death). However, the mBRS is very complex to remember and difficult to use in the daily clinical practice. In a previous study an easy-to-remember and easy to obtain simplified Predictive Model (sPM) has shown a good yield for predict rebleeding in patients with HRSU6 its utility for predict active bleeding has not been evaluated. The aim of this study is to evaluate the utility of the (sPM) to predict active bleeding in patients with gastroduodenal peptic ulcers.
Methods
We retrospectively reviewed the electronic and paper-based records of patients with gastroduodenal peptic ulcers whom underwent an endoscopy at the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán from February 2002 to September 2007. For this study, HRSU was defined as patients with hemorrhage from peptic ulcer disease (gastric or duodenal) with major bleeding stigmata, defined by groups according to Forrest's classification:7 Ia (spurting hemorrhage), Ib (oozing hemorrhage), IIa (non-bleeding visible vessel), and IIb (adherent clot). Patients with active bleeding correspond to Forrest Ia and Forrest 1b. Patients with hematemesis and those whom were hemodynamically unstable underwent an endoscopy after initial resuscitation. A regular diagnostic endoscope was initially used (GIF-100, GIF-130, GIF-140 or GIF-160, Olympus, Japan) and therapeutic modality (monotherapy or dual) was assigned according with physician criteria. Aside from epinephrine injection, endoscopic therapy (ET) was performed with heat probe coagulation, Argon plasma coagulation or Hemoclips (Olympus, Japan). All conscious patients were sedated with midazolam, phentanyl and/or propofol. Informed consent was obtained before the procedure in all patients.
Recurrent bleeding was clinically defined as the passage of hematemesis or melena, or both, coupled with the development of shock or decrease in hemoglobin concentration by at least 2 g/dL after initial stabilization of 24 hours or aspiration of fresh blood from nasogastric tube.8,9 Bleeding was confirmed by endoscopy or surgery in all cases. Clinical, laboratory, and demographic characteristics were recorded as well as, Forrest's classification, mBRS, the initial endoscopic technique for hemostasis, rebleeding, requirement for surgery, blood transfusion, and mortality during the first 30 days after the procedure. Monotherapy was defined as epinephrine injection alone on a 1:10 000 dilution. Dual therapy was considered when, besides epinephrine injection, an extra ET method was used (heat probe, argon-plasma coagulation, or hemoclips).
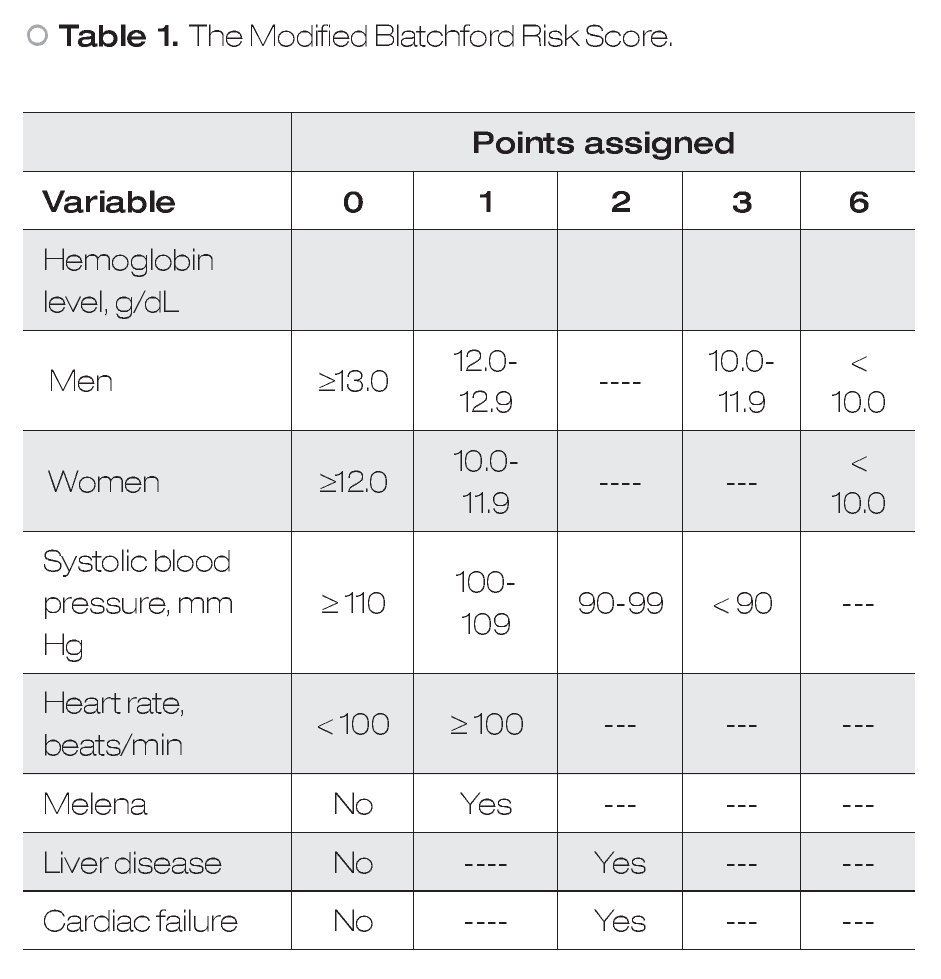
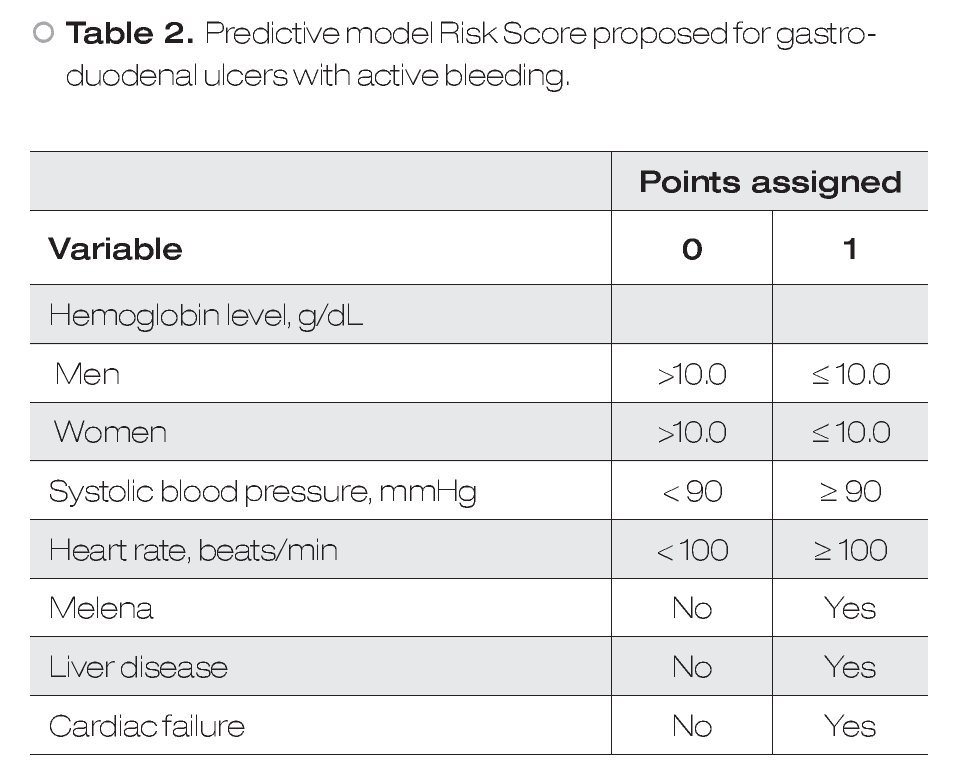
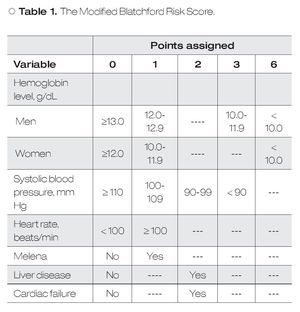
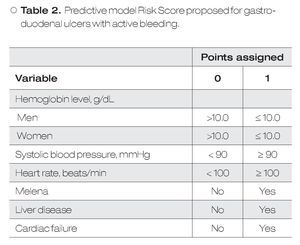
Predictive Models: According to the mBRS,5 different punctuation to hemoglobin levels were assigned as well as some clinical variables and comorbidities (Table 1), however, for final analysis patients were classified as a mBRS ≤ 1 (low risk) and patients with a mBRS > 1 (high risk), making useless these different punctuations. It is difficult to remember each one of the variables assigned to the mBRS in clinical practice. For this reason, in this work we only considered one cut-off point for each one of the variables to make easier for clinicians. Our sPM is on Table 2.6 We defined a low sPM/mBRS as ≤ 1.
Statistical analysis: Results are expressed as means and ± SD. Comparison of quantitative data was performed using the Student´s t-test. The differences between proportions of categorical data were obtained by the Fisher exact test when the number of expected subjects was less than five and by the Chi-square test otherwise. Predictive values were used to evaluate the utility of the model to predict active bleeding. Multivariate logistic regression models were used to assess the independent association between an mBRS of one or less and the occurrence of active bleeding or mortality. A p value < 0.05 was considered statistically significant. All statistical analyses were conducted using the statistics program SPSS/ PC version12.0 (Chicago, IL, USA).
Results
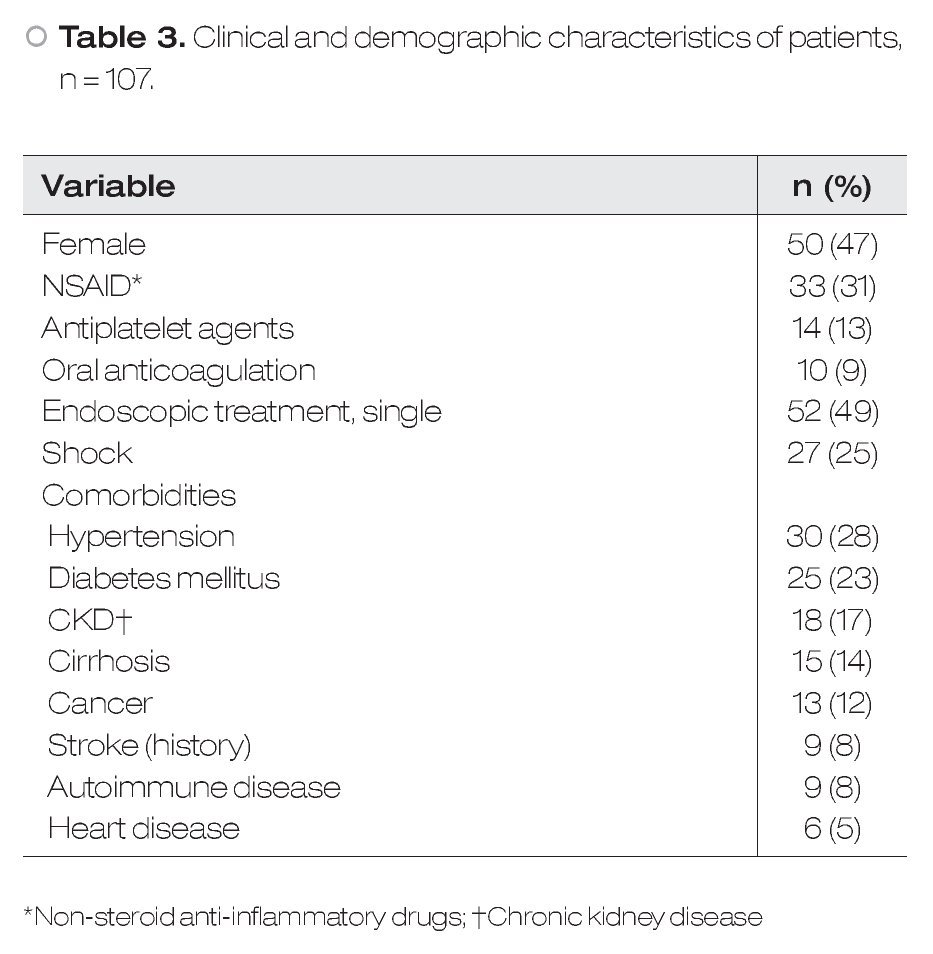
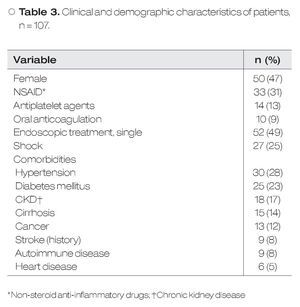
During the study period, 107 patients with HRSU were admitted to our hospital. Fifty women (46.7%) and fifty-seven men (53.3%) were included, with a mean age of 57.3 ± 17.1 years. The most common localization was the stomach, and Forrest IIa was the most frequent (39.3%) lesion. Clinical, demographic and laboratory characteristics are shown in Table 3. The mean number of endoscopic procedures by patient was 2 (range 1-4). Fifty (51%) patients received dual ET.
Initial success for endoscopic hemostasis was achieved in 98 patients (91.6%). Patients whom were not possible hemostasis with ET underwent surgery immediately. Four of them subsequently died (between one and twenty days after surgery). Recurrent bleeding was documented in 26/98 (26.5%) patients, with a median time of 2 (range 1-40) days. Besides the nine patients that required surgery due to lack of initial success, six patients underwent emergency surgery due to recurrent bleeding. Mortality was 18.7% (20 patients), although only in 7 patients the cause of death was related to ulcer bleeding (6.5%) with a follow up of 30 days.
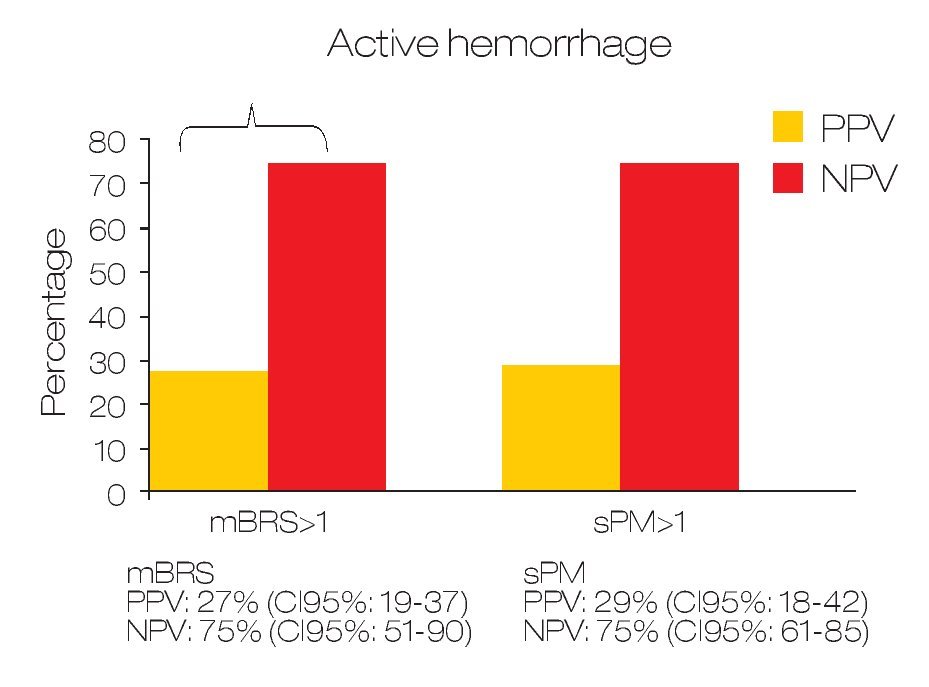
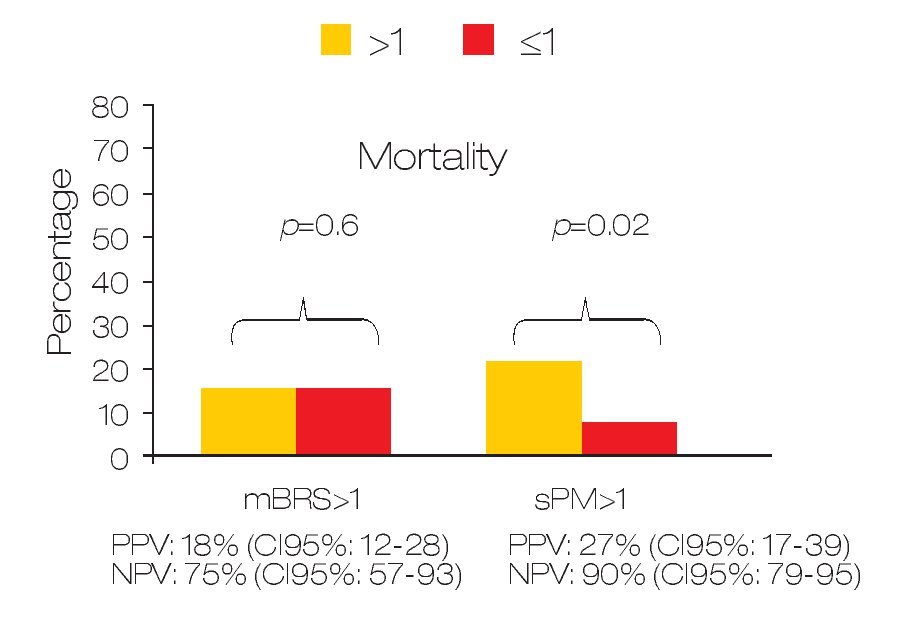
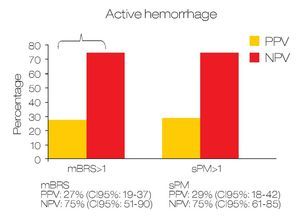
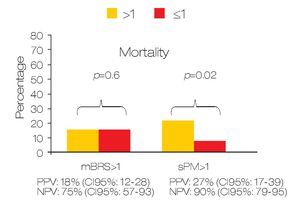
Predicting active bleeding: The positive and negative predictive values for active bleeding in patients with sPM >1 value was 28.6% (95% CI 18-42) and 75% (95% CI: 61-85) respectively. In the case of mBRS > 1 was 27.5% (95% CI 19-37) and 75% (95% CI 51-90) respectively (Figure 1). In multivariate analyses, only sPM was with statistical significance (OR: 1.80 [95%CI 1.1-2.9]; p = 0.018). For mortality, the positive and negative predictive value for patients with sPM >1 was 26.8% (95% CI 17-39.6) and 90.2% (95% CI 79-95.7) respectively. The positive and negative predictive values for mortality in patients with mBRS >1 was 18.7% (95% CI 12-28) and 81.3% (95% CI 57-93) respectively (Figure 2).
○ Figure 1.Comparison between sPM and mBRS for predict active bleeding in patients with gastroduodenal peptic ulcers.
○ Figure 2. Comparison between sPM and mBRS for predict mortality in patients with gastroduodenal peptic ulcers.
A low sPM was statistically associated with mortality (9.8% vs. 26.7%; p = 0.028) but low mBRS was not associated (18.7% vs. 18.6%; p = 0.6). The OR for mortality in patients with mBRS >1 was 0.99 (95% CI: 0.25-3.8) and OR for sPM > 1 was 3.3 (95% CI: 1.12-10).
Patients underwent to monotherapy (n = 52/107; 48.5%) do not have a higher mortality risk compared to patients underwent to dual endoscopic therapy (p = 0.32).
Discussion
In the present study we present that diagnostic yield of the sPM to role-out active bleeding in patients with HRSU is good. For our knowledge, this is the first study that evaluate the utility of sPM to role-out active bleeding. According with our results, the sPM is a useful parameter for role-out active bleeding and to predict mortality over mBRS. The sPM has some advantages over classical scores: 1) it is easier to obtain, 2) easier to use, and 3) it is easier to remember. Because the parameters used in sPM are mainly clinic ones and hemoglobin levels, we can say that this score is available everywhere. The sPM use simple punctuation (0 and 1) for every variable, so their use is very simple and practical. All parameters considered in the sPM are basic clinical parameters for critical patients and important comorbidities that always are considered in emergency patients. However, we have to keep in mind that we did not directly compared sPM vs mBRS in these points. In this study we considered two scores that, although similar, have some important differences between them: 1) the mBRS considers different punctuation to different hemoglobin levels as well as some clinical variables and comorbidities (Table 1); 2) in sPM only one cut-off point for each one of evaluated variables is considered. The differences observed between the two models to predict active bleeding and mortality are interesting. This could be explained because differences in given points for each variable in the two different scores (i.e. a patient only with liver disease, or cardiac disease, or SBP < 100 mmHg, or man with hemoglobin <12 g/ dL, are considered in high risk according to mBRS, whereas according to sPM these patients remain in the low-risk group). These data are in agreement with previous according to a good clinical utility of the sPM.6
Some limitations of our study are: 1) the endoscopic procedures were not videotaped, so we cannot reevaluate the accuracy of initial diagnosis (it is well known the relative poor inter-observer reliability of stigmata recognition);10 2) although the patients were enrolled as a prospective cohort, the conclusions of this study are limited by the fact that they are based on retrospective analysis; and 3) the medium sample size.
In conclusion, within patients with HRSU those patients with sPM ≤ 1 exhibit a low chance of active bleeding during endoscopy. More studies with a prospective design and a larger sample size are necessary to corroborate these findings.
Correspondence: Félix I Téllez-Ávila, MD.
Vasco de Quiroga N°15. Col.
Sección XVI. Tlalpan, 14000. Mexico City, Mexico.
Phone: (+525) 55487 0900, ext: 2150.
E-mail: felixtelleza@gmail.com