Nucleic acid amplification techniques are becoming widely used for mycobacterial detection as they combine high sensitivity, high specificity, and the rapid turnaround of results. Among the different steps that participate in ensuring a reliable molecular detection method, DNA extraction is crucial.
The novel oligochromatographic assay, Speed-oligo™ Direct Mycobacterium Tuberculosis (SO-DMT) (Vircell S.L., Santa Fe, Granada, Spain), is based on PCR technology combined with a dipstick to detect the presence of Mycobacterium and specifically identify Mycobacterium tuberculosis complex (MTC) in clinical respiratory specimens.
In our previous study, we demonstrated a high sensitivity of SO-DMT in detection of Mycobacterium spp. in acid fast bacilli (AFB) smear-positive clinical respiratory specimens and a simultaneous differentiation from MTC species.1
In this study, we compared on performance of the SO-DMT assay, related to mycobacterial culture, by using two different nucleic acid extraction methods: the one manual included in the SO-DMT assay (Vircell-NXM) and another automated performed with the GXT DNA/RNA nucleic acid extraction kit (Hain Lifescience, Nehren, Germany) (Hain-ANXM).
The purpose of comparison was, in case of a high correlation of results, to only perform the automated Hain-ANXM extraction for the SO-DMT assay with the aim of a single nucleic acid extraction with less hands-on-time which could also be used for the Genotype® MTBDRplus test in case of MTC detection.
SO-DMT and culture were used to prospectively assay 81 fresh respiratory specimens from 81 patients with suspicion of mycobacterial disease from August 2012 till June 2013. However, 23 could only be extracted by one method (21 by Vircell-NXM and 2 by Hain-ANXM). For this reason, they were excluded from the study as both extraction methods could not be compared. Also, 27 frozen respiratory specimens (culture results: MTC [12], and Nontuberculous mycobacteria (NTM) [15]) were retrospectively evaluated with the SO-DMT. Thus, a total of 85 specimens were included in the study. Respiratory specimens were decontaminated, examined by auramine staining according with a method previously published2 and cultured following standard protocols.3,4 Generally, Mycobacteria were identified by GenoType™ Mycobacterium CM/AS assay (Hain Lifescience, Nehren, Germany) or BD MGIT™ TBc ID (Beckton Dickinson, USA). However, one NTM isolate included was finally identified as Mycobacterium terrae by sequence-based methods (Gregorio Marañon Hospital, Madrid (Spain)).
Two aliquots of 650μl of decontaminated specimens were maintained for a maximum of 7 days at 4°C until assayed with SO-DMT. In those cases where we observed a disagreement among the results obtained by the two nucleic acid extraction methods, we confirmed the discrepancy by repeating the whole test again using new nucleic acid extracts from the beginning. In all cases, the disagreement kept. For the statistical analysis, the SPSS 15.0 software was used.
Out of the 85 specimens, 81 (95.3%) were Acid-Fast Bacilli (AFB) smear-positive: 60 (70.6%) strong-positive (≥1–9 AFB/field [200×]) and 21 (24.7%) weak-positive (<1–9 AFB/field [200×]). Finally, 4 (4.7%) AFB negative and mycobacterial culture positive specimens (1 MTC and 3 NTM) were included. According to the culture results, 61 (71.8%) were positive for MTC and 24 (28.2% for NTM). The NTM species isolated were identified as M. abscessus (5 [5.9%]), M. avium (10 [11.8%]), M. genavense (1 [1.2%]), M. gordonae (1 [1.2%]), M. intracellulare (5 [5.9%]), M. scrofulaceum (1 [1.2%]), and M. terrae (1 [1.2%]).
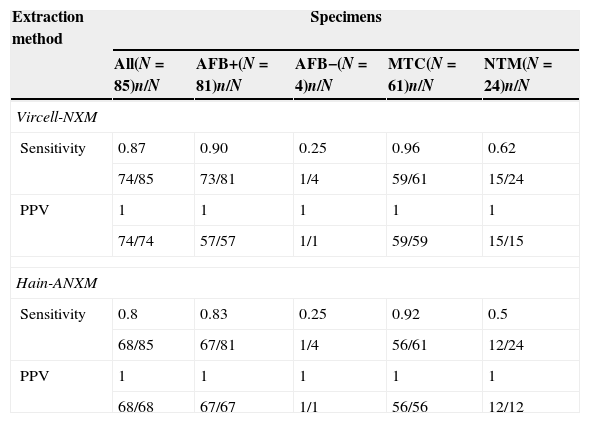
In the detection of Mycobacterium spp. in the 85 valuables specimens the Vircell-NXM showed an overall Sensitivity (S) of 0.87 (74/85) and Predictive Positive Value (PPV) of 1 (74/74); while Hain-ANXM showed a S of 0.8 (68/85) and PPV of 1 (68/68).
When considering the subgroup of 81 specimens AFB smear-positive; the S of the SO-DMT Vircell nucleic acid extraction method was 0.9 (73/81), (S: 0.95 (57/60) in AFB strong-positives and S: 0.76 (16/21) in the AFB weak-positives); whereas with the Hain-ANXM, S was 0.83 (67/81) (S: 0.92 (55/60) in the AFB strong-positive and S: 0.57 (12/21) in the AFB weak-positive subgroup) (Table 1).
Sensitivity and positive predictive values of SO-DMT assay compared to culture with both nucleic acid extraction methods studied.
| Extraction method | Specimens | ||||
|---|---|---|---|---|---|
| All(N=85)n/N | AFB+(N=81)n/N | AFB−(N=4)n/N | MTC(N=61)n/N | NTM(N=24)n/N | |
| Vircell-NXM | |||||
| Sensitivity | 0.87 | 0.90 | 0.25 | 0.96 | 0.62 |
| 74/85 | 73/81 | 1/4 | 59/61 | 15/24 | |
| PPV | 1 | 1 | 1 | 1 | 1 |
| 74/74 | 57/57 | 1/1 | 59/59 | 15/15 | |
| Hain-ANXM | |||||
| Sensitivity | 0.8 | 0.83 | 0.25 | 0.92 | 0.5 |
| 68/85 | 67/81 | 1/4 | 56/61 | 12/24 | |
| PPV | 1 | 1 | 1 | 1 | 1 |
| 68/68 | 67/67 | 1/1 | 56/56 | 12/12 | |
PPV: positive predictive value.
A total of 3 (2.9%) specimens gave a low detection signal for MTC (MTC +/−) while 2 (1.9%) specimens gave a low detection signal for NTM (NTM +/−) when Vircell-NXM was used. When Hain-ANXM was applied, a low detection signal for MTC (MTC +/−) and NTM (NTM +/−) was observed in 6 (5.8%) and 2 (1.9%) specimens respectively.
None of the nucleic acid extraction methods used with the SO-DMT assay showed molecular detection of NTM in a MTC case or vice versa.
In six specimens, four of them AFB weak-positive, no mycobacterial detection was observed with the automated extraction method. Meanwhile, in those six cases, the manual one showed a SO-DMT positive result (3 MTC and 3 NTM). But, in three out of these six cases (2 MTC and 1 NTM), the manual method gave a low detection signal. In accordance with previously exposed, our results showed a most efficient DNA extraction with the manual Vircell-NXM in comparison with the automated method. Specially, it happened in weakly positive AFB specimens. Moreover, the detection of mycobacterial DNA in AFB negative smear showed a very low S (S: 0.25) when we used any of those extraction methods. In any case, the SO-DMT assay showed a positive result only with the Hain-ANXM.
The manual extraction method took approximately 1h per specimen, which 15min were hands-on time to be distributed across the whole time period. Whereas the automated extraction method took also 1h per specimen, in which only 3min were hands-on time at the beginning of the process.
No previous study has compared the influences of two DNA extraction methods (a manual and an automated one) on the sensitivity of PCR amplification of the SO-DMT assay.
The main advantage of the automated nucleic acid extraction method is that it only required 3min hands-on-time, being the rest of the time fully automated.
However, the S is more important than laboratory automatization or economy in the diagnosis of tuberculosis. The automated system missed two specimens even among strong positive specimens.
There is a limitation in our study as we have not compared the manual and the automated extraction method with the SO-DMT in another kind of specimen different from the respiratory one.
Further studies will be necessary to assess the efficiency of both extraction methods with the SO-DMT assay when using specimens not belonging to the respiratory tract in which mycobacterial isolates have also been described.
In conclusion, even though the automated extraction method requires only 3min hands-on-time, it should never be used alone, without the manual one, neither in strong positive nor in weak positive auramine staining specimens.
Conflict of interestNone of the authors have conflict of interest.
We are grateful to Pablo Mendoza (Vircell S.L., Santa Fe, Granada, Spain) for providing the Speed-oligo™ Direct Mycobacterium Tuberculosis kits for the study.