Introduction
A number of studies have indicated that anergic HIV-infected patients have a high risk for developing tuberculosis (TB)1,2, but diverse clinical assays have not conclusively demonstrated the advantages of establishing antituberculosis chemoprophylaxis in this patient population3-4. Thus, the initial recommendation to establish anti-tubercular chemoprophylaxis in HIV-infected patients with anergy5 was later rejected6 and, at present, neither delayed hypersensitivity skin tests7 nor chemoprophylaxis is carried out on these patients8.
Nevertheless, there is evidence that certain groups of anergic HIV-infected patients, such as prisoners, drug users and the homeless, can have a high risk of tuber culosis1,2,8,9. One observational study even demonstrated a reduction in the risk of TB when isoniazid chemoprophylaxis was used over a twelve-month period in HIV-infected patients with anergy10.
We present the results of a prospective clinical study. The main objective of this study was to determine the tolerance to, and efficacy for TB prevention of three chemoprophylactic regimens in HIV-infected patients with anergy.
Methods
Design and study population
This study is a controlled, open, randomized, multi-center clinical assay, undertaken between 1 June 1994 and 31 December 1998 in twelve public hospitals, one located in Madrid and the remaining eleven in Andalusia. The study was authorized by the Clinical Research Ethics Committee of all participating centers and informed written consent was obtained from all the patients included. The criteria for enrollment in the study were as follows: 1) HIV infection confirmed by ELISA and Western blot; 2) age between 18 and 65 years; 3) life expectancy greater than two years; 4) cutaneous anergy defined by the absence of a reaction (0 mm) to skin reactivity tests with tuberculin, Candida albicans, and parotiditis antigens 72 hours after inoculation.
Testing for delayed cutaneous hypersensitivity was carried out on the inside of the lower left arm with the Mantoux method using: 0.1 ml of tuberculin (2 UI of PPD RT-23), 0.1 ml of Candida albicans antigen at a concentration of 1:10, and 0.1 ml of parotiditis antigen at a concentration of 1:10.000.
The exclusion criteria were as follows: 1) presence of active tuberculosis; 2) background of previous antituberculosis therapy or chemoprophylaxis; 3) presence of symptoms or signs suggesting pulmonary or extra-pulmonary tuberculosis; 4) history of hypersensitivity to the drugs used in the study (isoniazid, rifampin or pyrazinamide); 5) aspartate-aminotransferase and/or alanine-aminotransferase plasma concentrations more than or equal to four times their normal values, total bilirubin more than 2 mg/ml, and/or creatinine more than 2 mg/ml; 6) pregnancy; and 7) undergoing treatment incompatible with any of the drugs used in the study.
Randomization and interventions
The patients were randomly placed into one of the following four groups: isoniazid for 6 months (6H), rifampin plus isoniazid for 3 months (3RH), rifampin plus pyrazinamide for 2 months (2RZ) or no treatment (NT). The pharmaceuticals were administered daily by the patients themselves. The oral doses were as follows: isoniazid - 5 mg/kg/day (maximum 300 mg/day); rifampin - 10 mg/kg/day (maximum 600 mg/day); pyrazinamide - 1500 mg/day for patients weighing under 50 kg, 2000 mg/day for those weighing between 50 and 70 kg, and 2500 mg/day for those weighing more than 70 kg.
All of the participating subjects underwent a basal study that included clinical and epidemiological history, chest x-ray, hemogram, analysis of serum creatinine concentrations, BUN, uric acid, AST, ALT, alkaline phosphatase, and total bilirubin, as well as a CD4+ T-lymphocyte count.
During prophylactic treatment, patients were evaluated every 15 days for the first two months and monthly thereafter. At each check-up it was determined whether or not the patient was following the treatment properly and whether there were any adverse effects. A blood count and a biochemical study of hepatic and renal functions were also carried out at these visits.
The doctor in charge of each patient determined compliance to treatment by an oral interview. Therapeutic completion was defined as taking at least 80% of the total prescribed dosages. Prophylaxis was discontinued whenever a patient requested to do so or for any of the following reasons: appearance of Grade 3 or 4 side effects that could be attributed to the drugs used in the study; increases in AST and/or ALT values of three times or more their basal values; development of TB; or diagnosis of any disease that made interruption of the treatment advisable.
Evaluation and follow-up variables
The main variable of the study was development of TB. Confirmed TB was defined as isolation of Mycobacterium tuberculosis in any sample, probable TB as the presence of acid alcohol-resistant bacilli in any sample without confirmation or posterior identification in the cultures, and possible TB as clinically consistent disease that responded to treatment for tuberculosis. The other variables studied included suspension of prophylaxis (due to adverse effects, interactions between medications, or voluntary withdrawal) and mortality. Once prophylaxis was completed, clinical follow-up of the patients was performed for a two-year period, carrying out a study identical to the basal study every six months. In patients in whom TB was suspected, we performed the clinical tests required for its diagnosis, always including Ziehl-Neelsen staining and cultures in Lowenstein-Jensen media of fluids or tissue samples. If the latter were found to be positive, the procedure was to systematically identify the mycobacterium and perform sensitivity tests.
Sample size
The study was designed to have a sample size of 332 patients (83 patients per treatment group). The sample size was calculated assuming a confidence level of 95%, a power of 80%, an incidence of tuberculosis in the anergic patients not receiving treatment of 12.5% and an incidence of tuberculosis in the groups receiving treatment of 2%.
Statistical analysis
The analysis of continuous variables was carried out using the Student's t or the Mann-Whitney U tests. Chi-square or Fisher tests were used for the analysis of qualitative variables. The rate of TB per 100 person-years was calculated by dividing the number of cases of tuberculosis by the follow-up time of each subject. The relative risk of developing TB according to the chemoprophylaxis regimen was determined by calculating the reason for risk with a proportional risk regression or Cox regression method.
Results
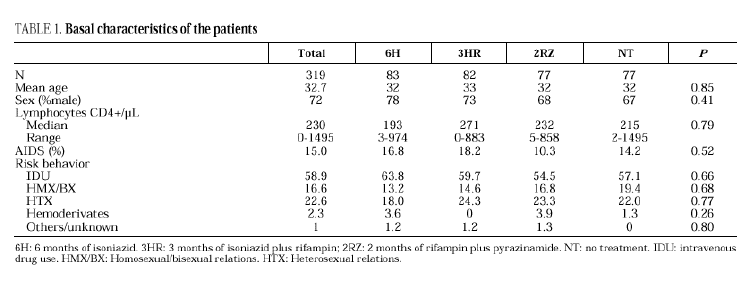
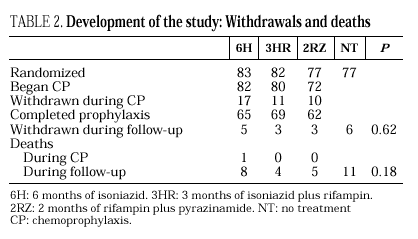
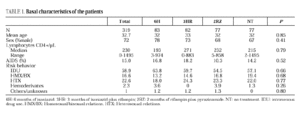
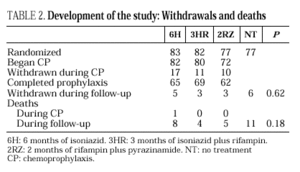
The study included 319 patients whose basal characteristics, described in Table 1, were comparable among the study groups. Following randomization, 10 patients did not begin chemoprophylaxis for the following reasons: diagnosis of active tuberculosis in 2 patients, Pneumocystis carinii pneumonia in 2, Mycobacterium avium intracellulare in 1, visceral leishmaniasis in 1, metrorrhagia in 1, pregnancy in 1, previous M. tuberculosis treatment in 1 and hyperbilirubinemia in 1. There were no differences among the groups regarding withdrawal during prophylaxis or follow-up (Table 2). Two patients undergoing methadone treatment who were assigned regimens containing rifampin voluntarily withdrew from the study so as to avoid adjusting the methadone dosage.
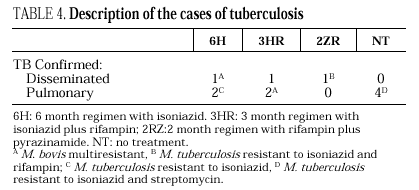
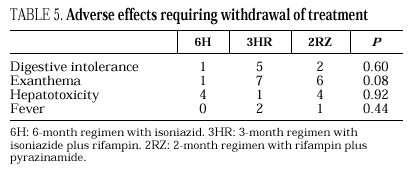
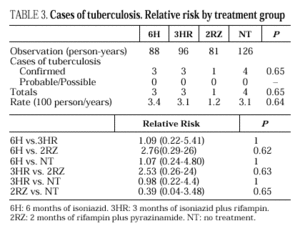
Eleven cases of TB occurred during follow-up, 3 in the 6H group, 3 in the 3RH group, 1 in the 2RZ group, and 4 in the NT group (p= 0.65); all were bacteriologically confirmed. Tuberculosis occurred on average at 14.5 months of follow-up (range 4-24 months; median 13 months), with no statistically significant differences among the groups. Follow-up was 88 (6H), 96 (3RH), 81 (2RZ) and 126 (NT) person-years of observation, respectively. The following rates of TB were found: 3.4 cases per 100 person-years in the 6H group, 3.1 cases per 100 person-years in the 3RH, 1.2 cases per 100 person-years in the 2RZ and 3.1 cases per 100 person-years in the NT (p= 0.64). The number of patients who developed TB, according to the prophylactic regimen and the relative risk with the confidence interval are shown in Table 3. The clinical and diagnostic characteristics of the patients with TB are shown in Table 4. Thirty-four patients experienced side effects that led to withdrawal of the medication, but there were no significant differences in this aspect among the treatment groups (Table 5). Of the 9 patients with hepatotoxicity, only one was removed from treatment (6H) due to symptomatic hepatitis. The remaining 8 were removed because of an asymptomatic increase in transaminases, defined as a level at least 3-fold higher than the basal value in the absence of hepatitis symptoms.
Twenty-nine patients died during the follow-up period from causes unrelated to TB. These included 6 cytomegalovirus, 4 terminal HIV disease, 3 sepsis, 2 cerebral toxoplasmosis, 2 progressive multifocal leukencephalopathy, 2 Pneumocystis carinii pneumonia, 2 heroine overdose, 2 lymphomas, 1 bacterial meningitis, 1 meningeal cryptococcosis, 1 respiratory insufficiency, 2 cerebral abscess, 1 Kaposi's sarcoma and 2 undetermined causes. Mortality was 14.3% in the group of patients not receiving treatment and 7.7% (18/234) in the treated group (p=0.08).
Discussion
HIV infection produces a significant defect in cell-mediated immunity that is evidenced in the inability to develop a delayed cutaneous hypersensitivity response to one or more antigens11. Because of this fact, the ability to diagnose latent infection by M. tuberculosis is diminished in many HIV-infected patients and there is a possibility that coinfection with M. tuberculosis may go undetected.
Study of cutaneous anergy is a simple and inexpensive way to evaluate immune function in HIV-infected patients12,13. One important aspect when assessing anergy is the criteria used to define it. We required an absence of reactivity to three antigens (PPD, C. albicans and parotiditis antigen) applied by the Mantoux method to define anergy. These criteria, recommended by international organizations14,15, have proven to be the most effective for defining cutaneous anergy in HIV-infected patients16.
Chemprophylaxis is highly effective for reducing tuberculosis in patients co-infected by HIV and M. tuberculosis4,17-19. A number of studies have demonstrated that the risk for developing tuberculosis in anergic HIV-infected patients is higher than in non-anergic patients who do not react to tuberculin20-23. One retrospective study carried out in Spain demonstrated that the risk might be even higher than in tuberculin-reactive HIV-infected patients who do not receive chemoprophylaxis2.
In the design of the present study carried out in the years 1993-1994, highly active antiretroviral treatment (HAART) was not taken into account. Therefore, the use of HAART during the follow-up period was not prospectively recorded and it was not possible to evaluate its effect on the incidence of tuberculosis. This is an important limitation of the study.
The incidence of tuberculosis in our group of anergic patients without chemoprophylaxis was 3.1 cases per 100 patient-years. The incidence of tuberculosis recorded in anergic HIV-infected patients depends on various factors, including the prevalence of TB in the area, the criteria used to define anergy, whether the study is prospective or retrospective, the degree of patient immunosuppression and the diagnostic criteria used to define tuberculosis. Our study found an incidence similar to that of other prospective studies carried out in areas with a high prevalence of tuberculosis, such as the one by Whalen et al4 carried out in Uganda (3.1 cases per 100 patient-years), and the one by Guelar et al20 carried out in Spain (2.6 cases per 100 patient-years). In contrast, our incidence is higher than that reported in studies from areas with a low rate of tuberculosis, such as the one by Gordin et al3 carried out in the USA (0.9 cases per 100 patient-years). The high incidence of tuberculosis in anergic patients with tuberculosis (12.4 cases per 100 patient-years) obtained by Moreno et al2 in a retrospective study carried out in Spain is striking. The incidence was even higher than that of tuberculin-reactive patients who were not receiving chemoprophylaxis in the same study. The fact that the study was retrospective and included a higher percentage of parenteral drug users (80%) and immunosuppressed patients (mean CD4+ lymphocyte count: 135/mm3), could explain the difference with respect to our results.
The main goal of our study was to evaluate the efficacy of three regimens of chemoprophylaxis for decreasing the incidence of tuberculosis in anergic HIV-infected patients. The first of the three regimens evaluated was 6H. In tuberculin-reactive subjects without HIV infection, this regimen reduced the incidence of tuberculosis by 69%24. In tuberculin-reactive patients with HIV infection, reported results are discrepant; Whalen et al4 demonstrated a 68% decrease in the incidence of tuberculosis with 6H, whereas Hawken et al19 found no differences with respect to the placebo. In anergic HIV-infected patients receiving 6H, two randomized clinical trials were unable to demonstrate a significant decrease in the risk of acquiring tuberculosis with respect to the placebo3,4, although in one of these studies tuberculosis risk decreased by 56%3. In the present study, the incidence of tuberculosis in patients receiving 6H was not lower than the incidence of tuberculosis in the non-treated group of patients.
The second of the evaluated regimens, 3RH, reduced the risk of tuberculosis in tuberculin-reactive HIV-infected patients by 60%4. However, this regimen has not been extensively evaluated in anergic patients. In one study conducted in Spain, 3RH showed an efficacy similar to 12H, but tuberculin-reactive patients as well as anergic patients were included, and equivalence between the two regimens was not demonstrated25. In our study, 3RH did not reduce the risk of tuberculosis with respect to the group of untreated patients.
The last of the regimens evaluated, 2RZ, has been considered one of the two regimens of choice for antituberculosis chemoprophylaxis in tuberculin-reactive HIV-infected patients9, since Gordin et al26 confirmed the results from a previous study27, showing that it was as efficient as the 12H regimen. In the present study, 2RZ reduced the risk of TB by 62%, but differences in risk with respect to the untreated group were not statistically significant. Cases of severe or fatal hepatitis have been reported in HIV-negative subjects receiving 2RZ as antituberculosis prophylaxis, making it unadvisable to treat such patients with this option28. In our study, as well as in other clinical trials26, the incidence of hepatoxicity among patients receiving 2RZ was not found to be higher. None of the patients participating in our study died due to toxic hepatitis, no significant differences in hepatoxicity were found among the three regimens of treatment, and the four patients treated with 2RZ who were withdrawn from the study because of hepatoxicity showed asymptomatic elevation of transaminases.
Development of exanthema is a well-known side effect of rifampin treatment that can lead to withdrawal of the drug29. In our study, exanthema was more frequent in patients receiving one of the two regimens containing rifampin, although differences were not statistically significant.
Mortality among the non-treated patients (14.3%) was higher than in the combination of the treated patients groups (7.7%). We have no explanation for this difference, even though it is slightly above the limit for statistical significance (p=0.08), and therefore, worthy of mention. The reason why prophylaxis decreases mortality in a given population is because it decreases the mortality provoked by the infection toward which it is directed. However, this was not verified in our study, since none of patients without prophylaxis died from tuberculosis.
In summary, although a 62% reduction in the incidence of tuberculosis was found in the 2RZ group with respect to untreated patients in our study, there was no significant reduction in the risk of tuberculosis with any of the three regimens evaluated. Therefore, our results do not support the use of chemoprophylaxis against tuberculosis in patients with skin anergy. However, the unexpectedly low rate of tuberculosis found in untreated anergic patients and the 62% reduction in TB incidence among patients treated with 2RZ when compared to the untreated patients despite this low rate, make it impossible to completely disregard the benefits of this strategy. In any case, the recommendations for avoiding tuberculosis in anergic HIV-infected patients should continue to be avoidance of exposure and, in the event that exposure should occur, initiation of appropriate chemoprophylaxis as soon as possible.
Our thanks to Dr. Agustín Gómez, from the Chamber of the Research Unit in the Hospital 12 de Octubre in Madrid for his help with the statistical analyses.