We present the case of a 41-year-old female patient who was admitted to the hospital at week 25 of her pregnancy for uterine dynamics and foetal lung maturity control. After five days of hospitalization, she began treatment with atosiban (oxytocin antagonist) due to increased uterine dynamics. Previous significant medical history presented in vitro fertilisation and gestational diabetes with insulin treatment. A cerclage was placed 22.3 weeks into the pregnancy due to prolonged cervical length.
During the hospitalization (week 27.5), the patient began experiencing an increased clear vaginal discharge without uterine dynamics resulting in a premature rupture of membranes. Laboratory blood test revealed an increase in C-reactive protein of 5.47mg/dL (0–0.5mg/dL). Based on the findings, a presumptive diagnosis of chorioamnionitis was made leading to amniocentesis for study of amniotic fluid.
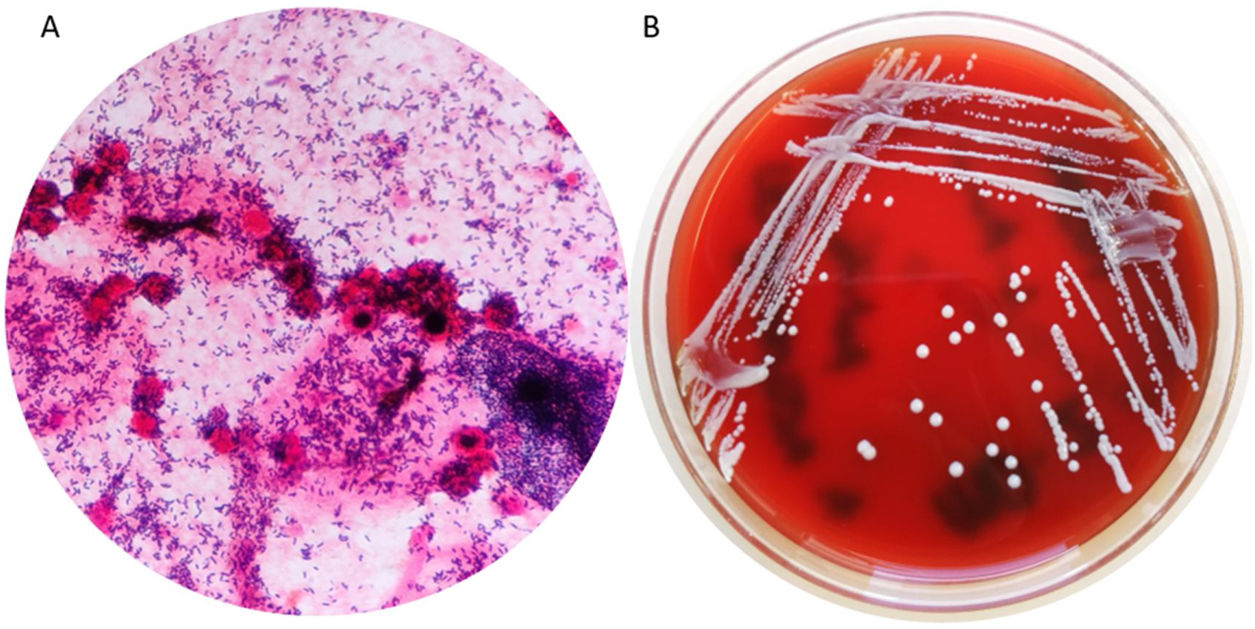
The results of the amniotic fluid study were as follows: glucose<2mg/dL, proteins 4g/L and a count of 1720 nucleated cells/mm3 with a predominance of polimorfonuclear cells. The Gram stain showed unbranched Gram-positive bacilli (Fig. 1).
Based on the laboratory data, the patient was diagnosed with chorioamnionitis starting with antibiotic dosage (intravenous piperacillin-tazobactam and oral clarithromycin). The obstetrician decided to induce labour and stopped the atosiban bolus. A baby weighing 1200g was born by spontaneous delivery at week 28.
The anatomical pathology study of the placenta showed acute necrotizing chorioamnionitis but the patient did not exhibit signs of fever or sepsis after delivery, and the antibiotic treatment was concluded 24h postpartum. Due to the premature birth, the new-born was admitted to paediatric intensive care and was treated with ampicillin and gentamicin. Antibiotic treatment concluded after 5 days without further signs of bacterial infection.
Microbiological studies used amniotic fluid and two placenta samples. All the samples were cultured in selective (MacConkey and Columbia CNA agar) and enrichment media (chocolate agar, blood agar, and thioglicolate broth) in aerobic conditions. We also used Anaerobe Schaedler and Schaedler neomycin vancomycin agar under anaerobic conditions (bioMérieux®). Two types of colonies were isolated in the amniotic fluid culture. Some colonies had a circular and smooth white colour and were identified as Actinomyces neuii by mass spectrometry (log score: +2.06, MALDI-TOF MS, Microflex®, Bruker). The other colonies were smaller and darker and were identified as Streptococcus anginosus by MALDI-TOF MS (log score: +2.16) as well. In both placenta samples, only Actinomyces neuii was isolated and identified (both log score: +2.16) (Fig. 1).
Susceptibility testing used the minimum inhibitory concentration (MIC) microdilution method (Pos MICroSTREP plus 6 panel, MicroScan WalkAway, Beckman Coulter). The sensitivity analysis showed that both strains were susceptible to all antibiotics tested: ampicillin A. neuii (≤0.06mg/L)/S. anginosus (0.12mg/L), cefotaxime (≤0.25mg/L), meropenem (≤0.25mg/L), levofloxacin A. neuii (1mg/L)/S. anginosus (≤0.5mg/L), clindamycin (≤0.06mg/L), clarithromycin (≤0.12mg/L), vancomycin (0.5mg/L), and daptomycin (≤0.25mg/L).
Streptococcus anginosus was not seen in the Gram stain, and it grew at low CFU counts in the inoculated amniotic fluid samples. The placenta samples did not reveal its presence; thus, it was considered to be a contaminant of the sample extraction.
Actinomyces neuii is a facultative anaerobic Gram-positive and rod-shaped bacteria. It has an unbranched morphology in contrast to other species of Actinomyces.1–3Actinomyces species are considered normal flora of the oral cavity, gastrointestinal tract, and female genital tract; they rarely cause disease in humans.3–8 Nevertheless, they can cause chronic and slowly progressive infections including abscess formation in skin and soft tissues.4,6,9Actinomyces neuii is a lesser-known species first described in 1994 by Funke et al.2 It does not cause typical actinomicosis. The infections are related to abscesses, skin lesions, and infections of the genitourinary tract and mostly associated with the use of intrauterine contraceptive devices (IUD).1,4,7–10 Rare cases of pericarditis, osteomyelitis, breast infections, ophthalmic infections, prostatitis, and bacteraemia have also been previously reported.1,3,5–8
Actinomyces neuii has also been involved in causing premature labour, chorioamnionitis, and neonatal sepsis associated with a previous resolution of a vaginal device (IUD or vaginal cerclage).1,4–10
In our patient, chorioamnionitis was suspected to be related to the cerclage placement at week 22 of pregnancy. Other authors reported similar conclusions of Actinomyces neuii infections associated with the use of this device.1,8 Only two cases reported the presence of Actinomyces neuii in amniotic fluid.5,10
This case demonstrates the need to consider Actinomyces infection in patients who carried a vaginal device. Amniotic fluid should be examined in patients with suspected chorioamnionitis for an early diagnosis to avoid further complications to the mother and the new-born.
Conflict of interestThe authors declare no conflict of interests.