Children undergoing chemotherapy for cancer have special vaccination needs after completion of the treatment. The aim of this study was to evaluate the adaptation of post-chemotherapy vaccination schedules.
MethodAn observational study was performed on a retrospective cohort that included all children aged from 0 to 14 years, who completed chemotherapy in a tertiary hospital between 2009 and 2015. Inclusion and exclusion criteria were applied. Immunisation was administered in accordance with the guidelines of the Vaccine Advisory Committee of the Spanish Association of Paediatrics. Primary Care immunisation and clinical records of the Preventive Medicine and Public Health Department were reviewed.
ResultsOf the 99 children who had received chemotherapy, 51 (70.6% males) were included in the study. As regards the type of tumour, 54.9% had a solid organ tumour, and 45.1% had a haematological tumour. Post-chemotherapy immunisation was administered to 70.6%. The most common vaccines received were: diphtheria-tetanus-pertussis or diphtheria-tetanus (54.9%), meningococcus C (41.2%), and seasonal influenza (39.2%). The rate of adaptation of the immunisation schedule after chemotherapy was 9.8%. The pneumococcal conjugate vaccine against 7v or 13v was administered to 21.6% of study subjects. However, only 17.6% received polysaccharide 23v. None received vaccination against hepatitis A. No statistically significant differences were observed between adherence to immunisation schedules and type of tumour (p=.066), gender (p=.304), or age (p=.342).
ConclusionPost-chemotherapy immunisation of children with cancer is poor. The participation of health professionals in training programmes and referral of paediatric cancer patients to Vaccine Units could improve the rate of schedule adaptation and proper immunisation of this population.
Los niños que son sometidos a quimioterapia en el contexto de un cáncer presentan necesidades especiales de vacunación una vez finalizado el tratamiento. El objetivo de este trabajo es evaluar la adaptación de los calendarios de vacunación posquimioterapia en una población pediátrica.
MétodoEstudio observacional de una cohorte retrospectiva. Se incluyeron todos los niños entre 0 y 14 años que recibieron quimioterapia en un hospital de tercer nivel entre 2009 y 2015. Se aplicaron criterios de inclusión/exclusión. Se siguieron las indicaciones oficiales del Comité Asesor de Vacunas de la Asociación Española de Pediatría para estas situaciones. Se consultó el registro de vacunación de Atención Primaria y el de la Unidad de Vacunas del Servicio de Medicina Preventiva y Salud Pública del centro sanitario.
ResultadosDe los 99 niños que recibieron quimioterapia, se incluyeron en el estudio 51. El 70,6% fueron varones. El 54,9% padecían un tumor de órgano sólido y el 45,1% un tumor hematológico. El 70,6% tenía registrada alguna vacuna tras el tratamiento. Las vacunas administradas con mayor frecuencia fueron: difteria-tétanos-tosferina o difteria-tétanos (54,9%), meningococo C (41,2%) y la gripe estacional (39,2%). La tasa de adaptación de calendario posquimioterapia fue del 9,8%. La vacuna frente a neumococo conjugada 7v o 13v fue administrada en el 21,6% de los niños evaluados, sin embargo, solo se completó con polisacárida 23v en el 17,6% de los casos. Ninguno recibió vacunación frente a hepatitis A. No se encontraron diferencias estadísticamente significativas entre el cumplimiento del calendario y el tipo de tumor (p=0,066), el sexo (p=0,304) o la edad (p=0,342).
ConclusiónExiste un importante margen de mejora en la adaptación de la vacunación posquimioterapia en niños con cáncer. La participación de los profesionales en programas de formación y la derivación de estos pacientes a las Unidades de Vacunas podría mejorar la tasa de adaptación garantizando una correcta inmunización en estos niños.
Immunisation is presently the main preventative strategy against infectious and contagious diseases.1 Systematic immunisation programmes for children have led to the eradication of diseases such as smallpox and the control of polio worldwide.2
Vaccination requirements are increasingly higher in special situations of both children and adults due to the recent increase in people with immune system alterations.3 The immunocompromised patient therefore needs a personalised immunisation schedule which will essentially vary according to the baseline disease, applied treatments and immunological status.4
It has been estimated that worldwide over 250,000 children are diagnosed with cancer every year, out of which it is calculated that 90,000 will die of the disease.5 In Spain, according to the Spanish Register of Childhood Tumours, the rate of childhood cancer is of 155.5 new cases annually per million children between the ages of 0 and 14.6 Mortality from cancer in childhood has decreased over 50% since the seventies.7 Chemotherapy is usually the treatment of choice in this type of disease. Its side effects depend on the type of drug, the dose and duration of treatment but in general these treatments cause a considerably high rate of immunodepression.8–10 During recent years treatments with chemotherapy have intensified, leading to high immunodepression in the patients and increasing the risk of losing immunity to previous vaccines admininstered.11 Once treatment has terminated these patients will therefore present with particular immunisation requirements being able to respond appropriately thanks to the complete recovery of humoral immunity, and the body's immune memory base.12
Given the above facts, the main aim of this research consists in assessing the adaptation of the immunisation schedule in children who received chemotherapy between 2009 and 2015 in an autonomous benchmark hospital. The secondary objective was to determine whether there were differences in immunisation in accordance with tumour type.
MethodsScope of the studyResearch was conducted in a third level hospital where occupation percentage was over 80% throughout the year. The Paediatric Oncology and Haematology Services were responsible for oncological child healthcare. The former was in charge of the patient with solid organ tumours (SOT) whilst the latter dealt with patients presenting with haematological tumours (HT). The cancer patient children received chemotherapy in the outpatient unit of the Day Hospital.
Study typeAn observational retrospective cohort study.
Inclusion/exclusion criteriaAll children aged between 0 and 14 who had received chemotherapy in the hospital between 1st January 2009 and 30th September2015 and who had been diagnosed with SOT or HT were included.
Exclusion criteria were:
- (a)
Having received a transplant from haematopoietic progenitors;
- (b)
Receiving active chemotherapy when assessed;
- (c)
Having terminated chemotherapy during the last 3 months of the assessment period;
- (d)
Receiving palliative treatment;
- (e)
Belonging to a different autonomous community;
- (f)
exitus prior to the termination of chemotherapy or during the first 3 months since termination;
- (g)
Relapsing during the first 3months since termination of chemotherapy with a new treatment initiation.
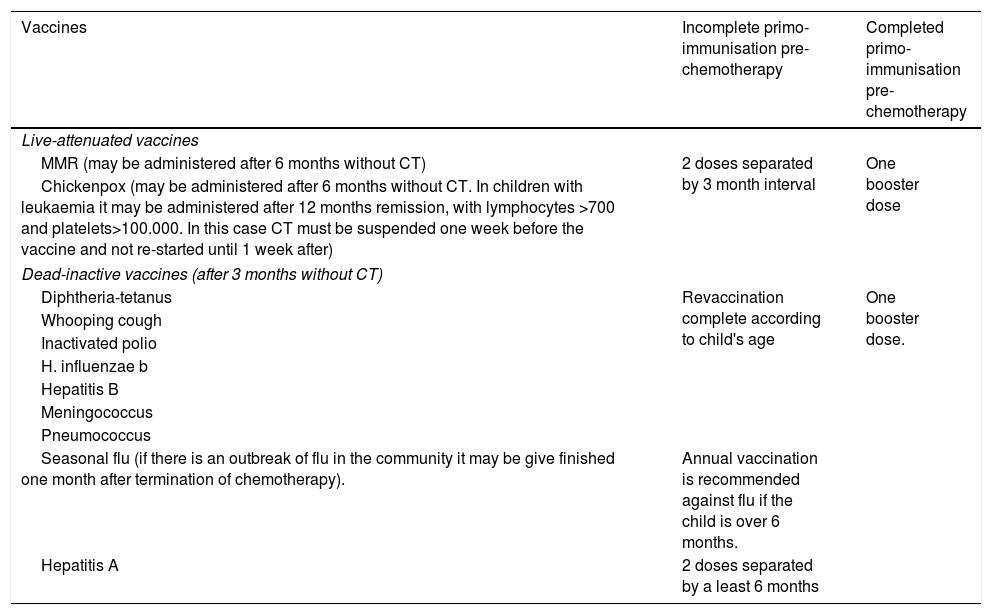
The on-line vaccine manual from the Vaccines Committee Assessor of the Spanish Paediatric Association was used as the main reference document.8 This document states that:
- -
In the case of children with cancer who have previously completed their first immunisation programme prior to the administration of the chemotherapy, it is recommended that a booster dose of all vaccines on the schedule be administered from 3 months in the case of inactive vaccines and from 6 months in the case of live vaccines. After this, the immunisation schedule should be continued according to the child's age.
- -
In the case of children who, on the contrary, have not completed their first immunisation programme prior to the chemotherapy treatment, it is recommended that complete re-vaccination be administered in accordance with the child's age.
Table 1 contains the immunisation guidelines in children with cancer, according to the previous instructions.
Vaccination guidelines in children with cancer, according to time of diagnosis.
| Vaccines | Incomplete primo-immunisation pre-chemotherapy | Completed primo-immunisation pre-chemotherapy |
|---|---|---|
| Live-attenuated vaccines | ||
| MMR (may be administered after 6 months without CT) | 2 doses separated by 3 month interval | One booster dose |
| Chickenpox (may be administered after 6 months without CT. In children with leukaemia it may be administered after 12 months remission, with lymphocytes >700 and platelets>100.000. In this case CT must be suspended one week before the vaccine and not re-started until 1 week after) | ||
| Dead-inactive vaccines (after 3 months without CT) | ||
| Diphtheria-tetanus | Revaccination complete according to child's age | One booster dose. |
| Whooping cough | ||
| Inactivated polio | ||
| H. influenzae b | ||
| Hepatitis B | ||
| Meningococcus | ||
| Pneumococcus | ||
| Seasonal flu (if there is an outbreak of flu in the community it may be give finished one month after termination of chemotherapy). | Annual vaccination is recommended against flu if the child is over 6 months. | |
| Hepatitis A | 2 doses separated by a least 6 months | |
Adapted from: (1) Moreno Pérez D, Nuñez Cuadros E. Vaccination en niños inmunodeprimidos o con tratamiento inmunosupresor. In: Asociación Española de Pediatría. Manual de la AEP 2012 de bolsillo. Madrid. Exlibris ediciones; 2012. 75–82. (2) Comité Asesor de Vaccines. Vaccination en niños inmunodeprimidos o con tratamiento inmunosupresor. Manual de vaccines en línea de la AEP [internet]. Madrid: AEP; 2015. Available in: http://vaccinesaep.org/documentos/manual/cap-14 [updated August 2015; accessed 15.02.16].
First immunisation or first vaccination is understood to be “a series of doses of the same natural biological product which must be administered to a susceptible person so that they achieve an appropriate immunity against the infection to be prevented”. It may also be called primary immunisation.13
We also requested the systematic child immunisation schedule records from the autonomous community under study from the Department of Health and Healthcare Services.
Lastly, we consulted the Primary Care Vaccination Record through electronic clinical records and the vaccination record of the Vaccines Unit of the Preventative Medicine and Public Health service of the centre.
Assessment strategyAssessment was conducted using the steps described below:
- (1)
Each child was assessed according to the systematic child immunisation schedule corresponding to their date of birth.
- (2)
The need for administration of a booster dose or complete revaccination was assessed according to the age and date of initiating chemotherapy.
- (3)
A relationship was established between the vaccines required according to previous criteria and vaccines registered in the electronic clinical records of Primary Care or the Vaccines Unit of the centre and which had been administered from 3 months after termination of chemotherapy treatment in the case of inactive vaccines and 6 months for live ones.
- (4)
To assess immunisation to chickenpox we took into account whether any doctor's record existed of the child having had this disease in infancy or whether after termination of chemotherapy there were laboratory tests which confirmed protective titres of antibodies (Immunoglobulin G chickenpox>150UA/ml).
- (5)
To assess immunisation to human papilloma virus in children the date of birth and age when chemotherapy was terminated was taken into consideration because this vaccine was introduced into the systematic autonomous child schedule in the year 2009 and corresponded to girls who had been born in 1995.
The rate of adaptation to the schedule after chemotherapy was measured in terms of the “% of adapted schedules=(Number of correct schedules between 2009 and 2015/total number of children assessed between 2009 and 2015)×100″. Correct schedule is understood to be that which reflects: the administration of 100% of the vaccines required according to the year of birth and the need for booster or re-vaccination.
The compliance rate according to vaccination type in terms of “% of vaccine adaptation X=(Number of children who received vaccine X between 2009 and 2015/total number of children for whom the administration of vaccine X was indicated between 2009 and 2015)×100″.
Although the conjugated vaccine 13v against pneumococcal infection was introduced into the autonomous community systematic child immunisation schedule in 2015 it was considered relevant to take it into consideration in the calculation of the compliance rate according to vaccination type since it is indicated in children and adults of any age who have received chemotherapy or immunosuppressant treatment.8,14 The polysaccharide vaccine 23v against pneumococcal infection was also considered in these patients since its administration is recommended in children from 2 years of age upwards in special situations.15
Lastly the vaccine against hepatitis A must be administered in these children because many of the immunosuppressant treatment have a great diversity of hepatotoxicity and infection during treatment could be severe.8
Ethical aspectsThe study was approved by the Ethical Committee of Autonomous Region Research (No. 130/15) and the Juvenile Prosecution Service was informed.
Statistical analysisDescriptive statistical analysis was made of every variable (univariant analysis), expressing the absolute and relative frequencies of the qualitative variables researched. The mean was calculated as a central tendency measurement and as standard deviation. Vibrant analysis was performed to determine whether the study variables selected were associated or not. For the qualitative variables the exact Fisher test and the Chi-square test was used. The non parametric Mann–Whitney U test was used as the quantitative variables analysed did not follow a normal distribution according to the Shapiro–Wilks test (p<.001 in the 4 assessment criteria and in the total assessment) and the sample size was under 30 subjects in one of the groups. Statistical significance was considered to be when p<.05. Version 18.0 of the Statistical Package for the Social Sciences was used.
ResultsGeneral sample descriptionA total of 99 children who received chemotherapy between January 2009 and September 2015 were identified. Of them 48 were excluded after the application of the exclusion criteria, with the main causes being: exitus prior to the termination of treatment or during the first 3months after it (35.41%), transplant from haematopoietic progenitors (29.16%), active chemotherapy or in the last 3 months (27.08%), belonging to another autonomous community (6.25%) and palliative treatment (2.08%). The final study sample included 51 children of whom at diagnosis, 33.3% were aged between 0 and 4, 39.4% between 5 and 9 and 27.3% between 10 and 14.
The general description was as follows: 70.6% (36) were boys and 29.4% (15) were girls; 54.9% (28) had SOT and 45.1% (23) HT. The mean and standard deviation of age of diagnosis and initiation of chemotherapy was 7.5 (±4.3) and 7.6 years of age (±4.3), respectively. No significant differences were found between both ages and tumour type (p=.080; p=.127).
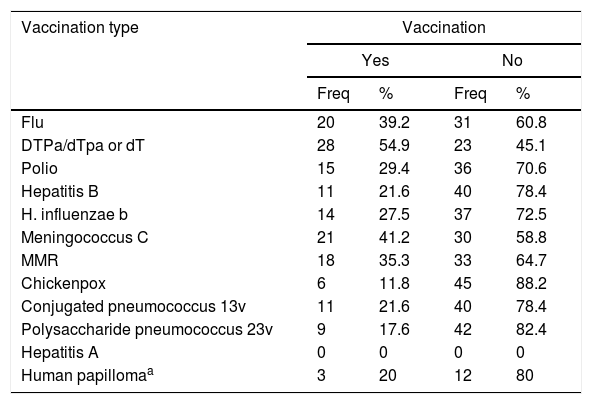
General description of recorded immunisationWith regard to post-chemotherapy immunisation, 70.6% (36) had recorded the administration of a vaccine once treatment had finalised whilst 29.4% (15) did not have any type of registration of vaccination after immunosuppression. The reasons for non vaccination were not recorded in any of these cases. Table 2 shows that, depending on age, the diphtheria-tetanus-pertussis vaccine (DTPa/dTpa) or diphtheria-tetanus (dT) was the most frequently administered (54.9%), followed by the vaccine against meningococcus C (41.2%) and seasonal flu (39.2%).
Frequency and percentage of vaccination according to vaccination type.
| Vaccination type | Vaccination | |||
|---|---|---|---|---|
| Yes | No | |||
| Freq | % | Freq | % | |
| Flu | 20 | 39.2 | 31 | 60.8 |
| DTPa/dTpa or dT | 28 | 54.9 | 23 | 45.1 |
| Polio | 15 | 29.4 | 36 | 70.6 |
| Hepatitis B | 11 | 21.6 | 40 | 78.4 |
| H. influenzae b | 14 | 27.5 | 37 | 72.5 |
| Meningococcus C | 21 | 41.2 | 30 | 58.8 |
| MMR | 18 | 35.3 | 33 | 64.7 |
| Chickenpox | 6 | 11.8 | 45 | 88.2 |
| Conjugated pneumococcus 13v | 11 | 21.6 | 40 | 78.4 |
| Polysaccharide pneumococcus 23v | 9 | 17.6 | 42 | 82.4 |
| Hepatitis A | 0 | 0 | 0 | 0 |
| Human papillomaa | 3 | 20 | 12 | 80 |
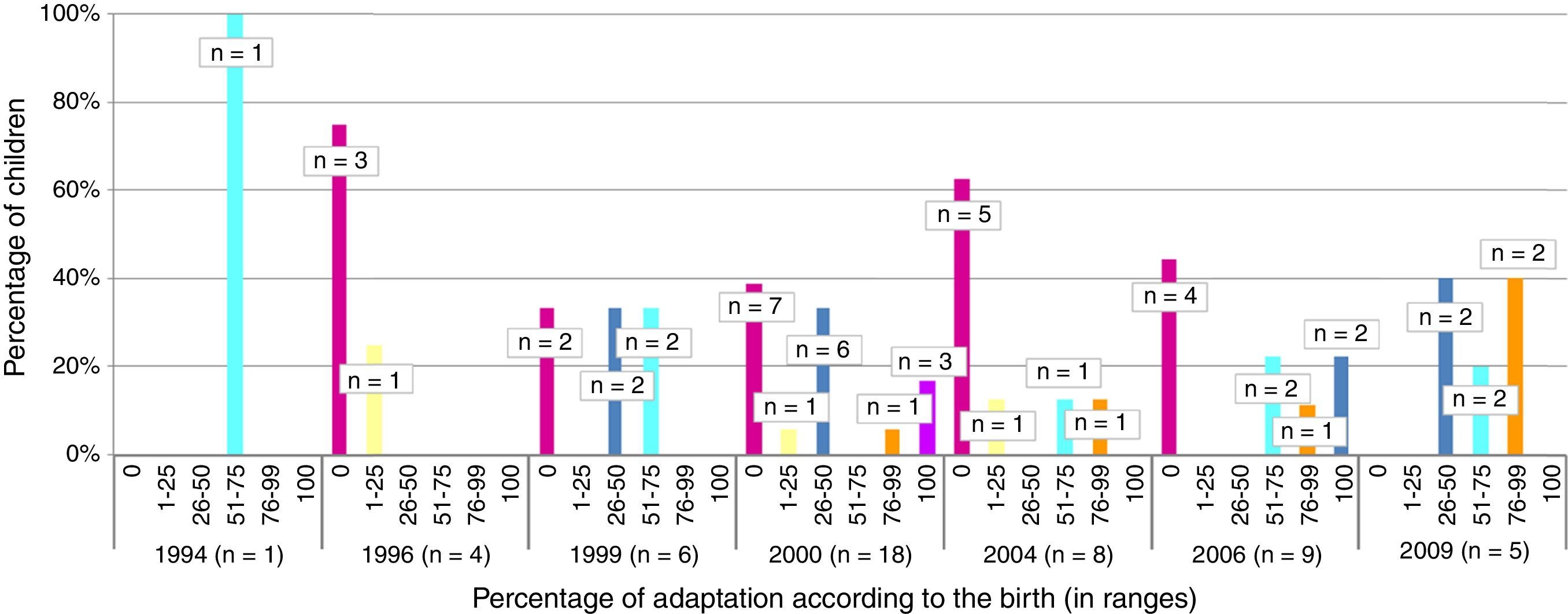
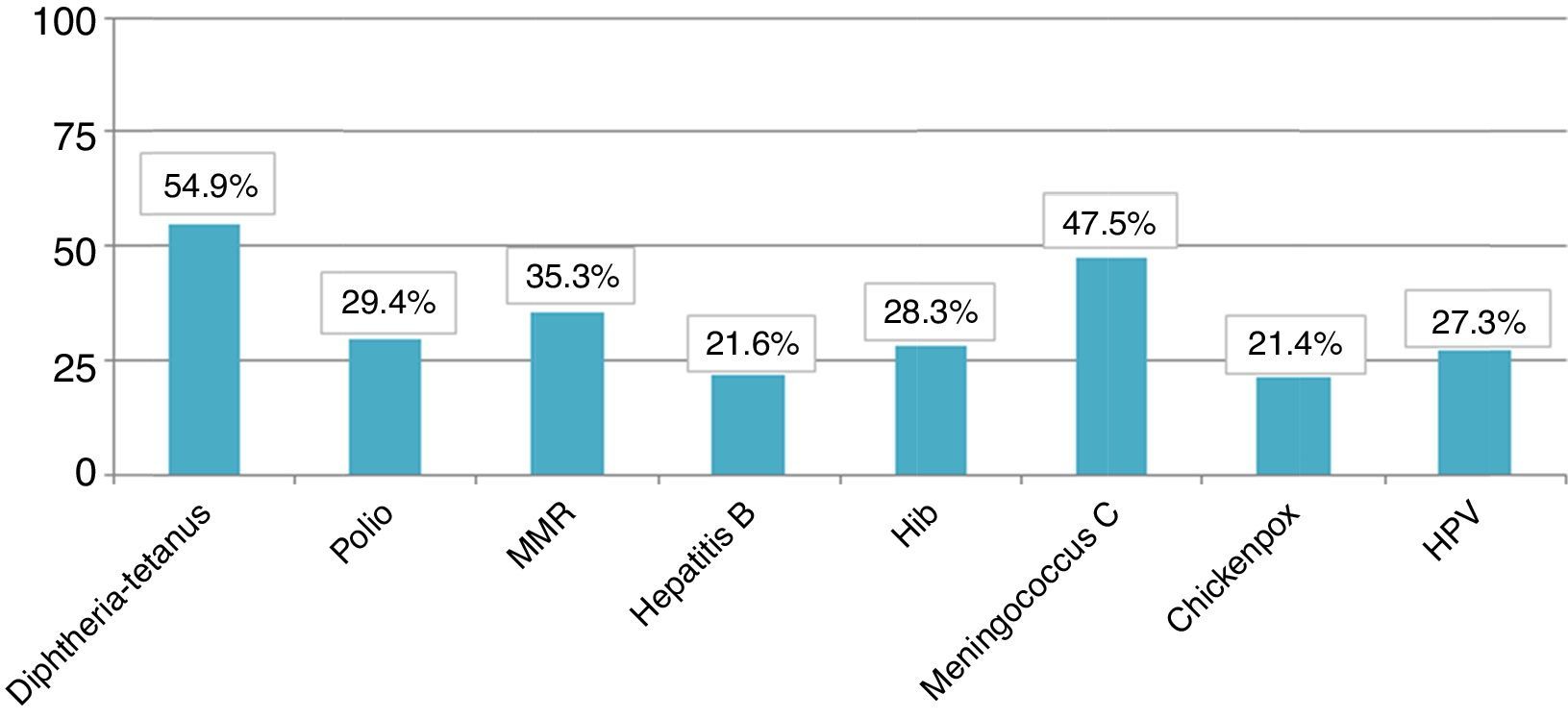
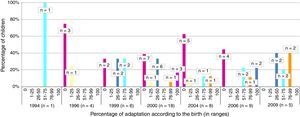
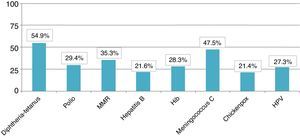
The total member of children who presented appropriate adaptation to the systematic immunisation schedule after chemotherapy was 5, comprising an adaptation rate of post-chemotherapy schedule of 9.8%. In Fig. 1 we may observe the percentage of adaptation of systematic vaccination after chemotherapy (in ranges of 25) in accordance with the corresponding vaccination schedule by year of birth and the absolute number of children in each group. Fig. 2 shows the rate of compliance according to the vaccination type and indication by year of birth. Only the vaccine for DTPa/dTpa or dT exceeded by 50% compliance according to indication, followed by meningococcus C (47.5%) and MMR (35.3%).
In 3 of the 51 children assessed the diagnosis of cancer was made before they were reached the age of one and primo vaccination was therefore not completed. They were then referred for revaccination in accordance with their age on finalising chemotherapy. None of these children were recorded with an appropriate adaptation to the immunisation schedule.
No significant differences were found between the adaptation of the schedule and tumour type (p=.066), patient gender (p=.304) or age in ranges (p=.342).
Vaccination against chickenpoxThis vaccine was included in the systematic child immunisation schedule of the autonomous community under study in 2006. The number of children assessed with indication of vaccination was 14, but only 3 (21.4%) received the vaccine. Of the remaining 11 only 2 (18.1%) presented with immunoglobulin G (+) after termination of chemotherapy. There were no records of others for laboratory testing or medical documentation of having suffered from the disease.
Vaccination against human papillomaThis vaccine was introduced into the autonomous systematic child schedule in 2009 for girls who had been born in 1995. Of the 15 girls assessed, 11 (73.3%) presented with indication of the vaccination against human papilloma virus, according to their year of birth and when chemotherapy was completed. However, it was observed that only 3 (26.6%) had received this vaccine.
Vaccination against pneumococcusThe systematic vaccination against pneumococcus was included in the schedule for children born from 1st January 2015 onwards in the autonomous community under study and therefore indication of vaccination in these patients was not linked to the schedule but to their immunodepressant status. The vaccine against conjugated pneumococcus 7v or 13v was therefore administered to 21.6% of children but was only completed with the polysaccharide 23v vaccine in 17.6% of cases.
Vaccination against hepatitis ANo child received vaccination against hepatitis A after termination of treatment.
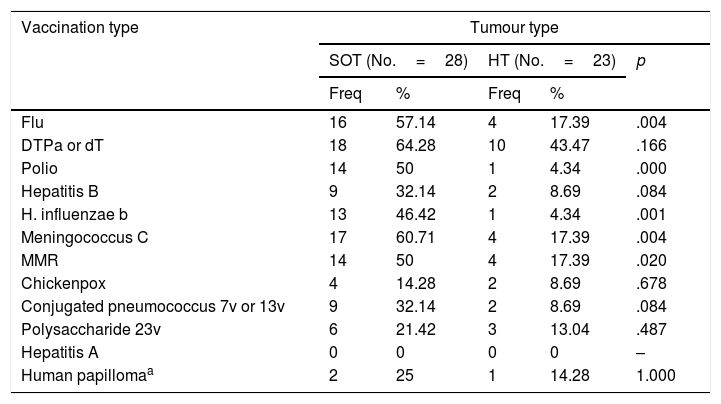
Vaccines received according to tumour typeStatistically significant differences were found between the post chemotherapy vaccination of children with SOT and HT for several vaccines, in favour of the children with SOT (Table 3).
Frequency, percentage and p-value of vaccination according to vaccination type and tumour type. 1=calculated only for the group of girls (SOT No.=8; HT No.=7).
| Vaccination type | Tumour type | ||||
|---|---|---|---|---|---|
| SOT (No.=28) | HT (No.=23) | p | |||
| Freq | % | Freq | % | ||
| Flu | 16 | 57.14 | 4 | 17.39 | .004 |
| DTPa or dT | 18 | 64.28 | 10 | 43.47 | .166 |
| Polio | 14 | 50 | 1 | 4.34 | .000 |
| Hepatitis B | 9 | 32.14 | 2 | 8.69 | .084 |
| H. influenzae b | 13 | 46.42 | 1 | 4.34 | .001 |
| Meningococcus C | 17 | 60.71 | 4 | 17.39 | .004 |
| MMR | 14 | 50 | 4 | 17.39 | .020 |
| Chickenpox | 4 | 14.28 | 2 | 8.69 | .678 |
| Conjugated pneumococcus 7v or 13v | 9 | 32.14 | 2 | 8.69 | .084 |
| Polysaccharide 23v | 6 | 21.42 | 3 | 13.04 | .487 |
| Hepatitis A | 0 | 0 | 0 | 0 | – |
| Human papillomaa | 2 | 25 | 1 | 14.28 | 1.000 |
The need to adapt the immunisation schedules to groups at risk is increasingly higher. This includes children and adults in special situations and/or with chronic diseases with or without immunodepression.3,4 In general, it has been established that the time for diagnosis of the disease is the most ideal for carrying out this updating, since the immunological response to vaccines is higher.16
Specifically, paediatric age patients who have undergone chemotherapy treatment are a risk group.17 Adaptation to the child immunisation schedule adhering to the official recommendations becomes a major preventative tool as it may determine morbidity and mortality to a great degree.4,17,18 It should also come under consideration that Spain is receiving an increasingly larger immigrant and refugee population and there are therefore groups of people who have not been immunised against chickenpox and measles, for example. This means herd immunity dilution and an increase in the risk of immunodepressed people catching diseases.19
In this study we observed that the percentage of correctly adapted schedules after chemotherapy was lower than 10%. Despite there being numerous studies about the population at risk and the needs for vaccine adaptation4,8 there are few published works which cover vaccination in the paediatric population in special situations20 and even less of those which assess the adaptation of these schedules in children who have received chemotherapy.
With regard to compliance according to vaccination type, the 3 most frequently administered were the vaccine against meningococcal C, DTPa/dTpa or dT and the seasonal anti-flu vaccine. The anti-meningococcal C may be explained in relation to the introduction of this vaccine into the systematic child schedule of the autonomous community in 2000, owing to an epidemic outbreak the previous year.21 As a result, health service professionals are highly aware of this vaccine. Equally DTPa/dTpa or dT is normally administered due to accidents in the home.22 Lastly, due to the major consequences the flu virus infection could have in these patients, the seasonal flu inactive vaccine has been one of the most studied vaccines.23 This has been considered safe and immunogenic in this group24,25 despite the fact that several authors have found coverage under 25% in children in situations of risk.20
Systematic vaccines against chickenpox and human papilloma virus had the lowest compliance rate. At the time of research it was considered that the first should be administered at 10 years of age provided that the child had not previously had it or had not suffered from the disease. It is worth considering that the fact that only 11.5% of children assessed had received this vaccine could be due the existence of major under-recording in the medical records of having had the disease or low concern by professionals towards serological verification of titre antibody protectors after chemotherapy. In the case of the vaccine against human papilloma virus and its referral for administration at 13 years of age in the autonomous schedule, low compliance could be due to the fact that the girls who had finished chemotherapy after the age of 14 stopped being cared for by the paediatrician in primary care and went on to the GP who was probably less sensitive to the issue of this vaccine. The vaccine against hepatitis A was not administered to any of the patients perhaps because professionals were unaware of the risk of complications regarding infection during and after chemotherapy.
There are many authors who have demonstrated the benefits of the anti-pneumococcal vaccination in recent years in patients at risk.26–28 As a result it is known that in children with cancer the administration of 2 doses against conjugated pneumoccocus of 7 serotypes offers between 86% and 100% protector antibodies.29 Despite this, in this study we observed coverage of 21.6% for the conjugated pneumoccocus of 7 or 13 serotypes and 17.6% for the polysaccharide of 23 serotypes.
The results of our study indicate greater frequency of administration of some of the vaccines in children with SOT compared with children with HT. It is currently unknown what the main reason for this variability is, but it may be due to the type of specialist carrying out the follow-up. That is to say, children with SOT have follow-up by paediatricians whilst children with HT are followed up by haematologists, which could give rise to the latter confiding in the fact that the paediatric team will be responsible for adaptation to the immunisation of these patients. However, none of the groups stand out for having a better rate of adaptation to the schedule.
Lastly, it is of note that this study is not exempt from limitations. On the one hand, after the application of the exclusion criteria, a total of 51 children out of 99 were initially selected. There is no doubt that the sample could have been larger if the temporary range had been extended but no exacting hospital records prior to these dates were available. It could otherwise have been the case that under-registration took place in the electronic medical records of Primary Healthcare or in the Hospital Immunisation Unit. Notwithstanding, and taking into account the systematic child vaccination records we believe this was not the case.
To conclude, compliance in adaptation of the immunisation schedule in children who have received chemotherapy is low. Involvement by the hospital physician responsible and primary care in updating post-chemotherapy immunisation is essential for raising post-treatment immunisation cover in this risk group. Communication between both and with the GP when the patient is at the age limit of paediatric care will facilitate compliance in this matter, when necessary. Furthermore, the referral of these children to the Immunisation Unit of the preventative Medical and Public Health Services will guarantee correct continuity of medical and nursing care in these matters by providing close follow-up of the child's immunisation throughout the tumour process (immunisation programming in terms of ideal moment, number of doses, posterior serological confirmation, documentary record, etc.). Continuous training in immunisation should be a constant presence in the healthcare environment.
FinancingThis study received no financing by any public or private entity.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Fernández-Prada M, Rodríguez-Martínez M, García-García R, García-Corte MD, Martínez-Ortega C. Adaptación de los calendarios de vacunación en población pediátrica que ha recibido quimioterapia. Enferm Infecc Microbiol Clin. 2018;36:78–83.