Pertussis is a highly contagious, vaccine-preventable respiratory tract infection, with high morbidity and mortality and a particularly severe effect on newborns and infants under 2 months. The first pertussis vaccines were introduced in the 1940s. Since 1980, however, the incidence of cases has risen despite the extensive vaccination programmes and antibiotic adjuvant treatments available. Transition from the use of whole-cell vaccines to acellular vaccines and the antigenic modifications of Bordetella pertussis have contributed, among other factors, to a reduction in vaccine-acquired immunity and reemergence of the disease. Today, there are still unmet needs not covered by conventional prevention measures and existing antibiotic treatments. This review aims to update the available data, and to discuss which vaccine strategies might contribute to better disease control and prevention.
La tosferina es una infección respiratoria inmunoprevenible altamente contagiosa, con alta morbilidad y mortalidad, y que afecta con especial gravedad a recién nacidos y lactantes menores de 2 meses. Las primeras vacunas comenzaron a emplearse en la década de los 40. Sin embargo, desde 1980 la incidencia de casos ha aumentado a pesar de los amplios programas de vacunación y tratamientos antibióticos adyuvantes disponibles. El cambio del uso de vacunas celulares a vacunas acelulares, y las modificaciones antigénicas de B. pertussis han podido contribuir entre otros factores a la disminución de la inmunidad adquirida tras la vacunación y a la reemergencia de la enfermedad. En la actualidad, todavía existen necesidades no cubiertas por las medidas convencionales de prevención y los tratamientos antibióticos existentes. Esta revisión pretende actualizar los datos disponibles y plantear qué estrategias vacunales pueden contribuir a un mejor control y prevención global de la enfermedad.
The main aetiological agent of pertussis (also known as whooping cough) is Bordetella pertussis, a gram-negative coccobacillus with a strictly human reservoir and high airborne transmissibility. Pertussis is an acute respiratory infection characterized by a violent cough that can last for weeks or months and is seen mostly in the preschool and school-age population. Although adolescents and adults are the main reservoir of the microorganism and play a crucial role in its transmission, it is infants under 4 months who have the highest incidence and most severe forms of the disease, which often requires advanced life support. In the last 20 years, a reemergence of the infection has been observed, making it one of the most prevalent vaccine-preventable diseases. The appearance of B. pertussis strains that escape the immunity conferred by the vaccine and the gradual reduction of the protection acquired after vaccination are among the possible causes of this reemergence. These situations justify the need to adapt the current preventive, diagnostic and therapeutic strategies with the primary aim of protecting the most vulnerable population (infants<4 months) and reducing the rising trend in the incidence of pertussis in the general population. This review updates the epidemiological situation of pertussis, the microbiological aspects related to the adaptation of B. pertussis and the diagnosis of the disease, and the preventive actions related to existing vaccine strategies that may help to improve its control and prevention.
Epidemiological situation of pertussisThe European Union (EU) network for epidemiological surveillance and control of vaccine-preventable infections estimates that 35627 cases of pertussis were reported by the participating countries in 2018, with a notification rate of 8.2 cases/100000 population, similar to that of the previous 4 years but the lowest observed in the same time period.1 Five countries (Germany, the Netherlands, Norway, Spain and the United Kingdom) accounted for 72% of all notified cases, and 62% of cases were individuals aged over 15 years. Infants under 1 year, who had not completed the primary pertussis vaccination series because of their young age, were the most affected age group with the highest incidence rate (44.4 cases/100000 population), followed by children aged 10–14 years.
The epidemiological evolution of the infection in adolescents and adults is of particular concern because of their potential to transmit the bacteria to infants. In adults, clinical suspicion of pertussis is low, which may explain the significant underreporting of cases.2 The review of the definition of pertussis cases by the EU in 2018 probably helped to identify atypical presentations of the disease in adults, adolescents, and vaccinated individuals, and to clarify aspects of laboratory confirmation.3,4
Most EU states report pertussis cases according to the Community definition, based on extensive passive surveillance systems with national coverage. Even so, the form of notification is heterogeneous. Belgium uses a laboratory-based, voluntary sentinel surveillance system covering the entire population, while France has a hospital-based sentinel surveillance system, which includes only infants under 6 months. There are two surveillance systems in Denmark: one laboratory-based (which includes all age groups) and another in which doctors must report pertussis cases in children under 2 years.
In Spain, pertussis has been a notifiable disease since 1982 and individually notifiable since 1997. Passive epidemiological surveillance systems have significant problems of underdiagnosis, not only in adults, but also in children.2 Underreporting of symptomatic cases is considerable in children ≥1 year, although the highest percentage of underreporting (50%) has been identified in infants under 1 year. In the period 1997–2010, the overall reported incidence of hospitalization for pertussis was 1.3 cases/100000 population in children and adults. Minimum underreporting ranged from 3.8% to 22.8%, according to the study year.5 These data support the need for rapid laboratory diagnostic tests and greater surveillance of family contacts of any age that help to better quantify existing cases. Intensified surveillance may also be useful for a better understanding of pertussis transmission patterns in the family and community. Surveillance and monitoring of pertussis incidence and outbreaks can be improved by standardizing and strengthening regional epidemiological surveillance systems with the use of complementary active systems that allow actual incidence data to be determined and by introducing innovative approaches (such as Big Data analysis, based on real-time tracking of Internet search activities).6,7
Pertussis incidence in SpainThe incidence of pertussis has increased significantly in Spain since 2011. The highest rates are recorded in infants under 1 year, followed by children aged 5–9 years, as observed in Catalonia, Galicia, Madrid and Valencia and in outbreaks in Andalusia, Valencia, and Catalonia.8–14
Despite high vaccination coverage, pertussis maintains its epidemic presentation. Between 1998 and 2018, five epidemic waves of pertussis have been described. Since 2010, there has been a sustained epidemic that retains the cyclical pattern, although at a level higher than in previous years. The incidence has been increasing in infants under 1 year, who continue to be the most affected. Incidence rates (IRs) per 100000 population were 150.6 and 119.5 cases in 2017 and 2018, respectively, followed by IRs for children aged 1–4 years (56.7 and 39.5 in 2017 and 2018, respectively), 5–9 years (62.3 and 30.3), and 10–14 years (37.1 and 33.0).15 These data suggest a progressive accumulation of individuals susceptible to infection due to decreased immunity after years of low incidence and highlight the need for new vaccination strategies and more effective vaccines to prevent pertussis in unvaccinated children, in whom the disease may present more severely.16
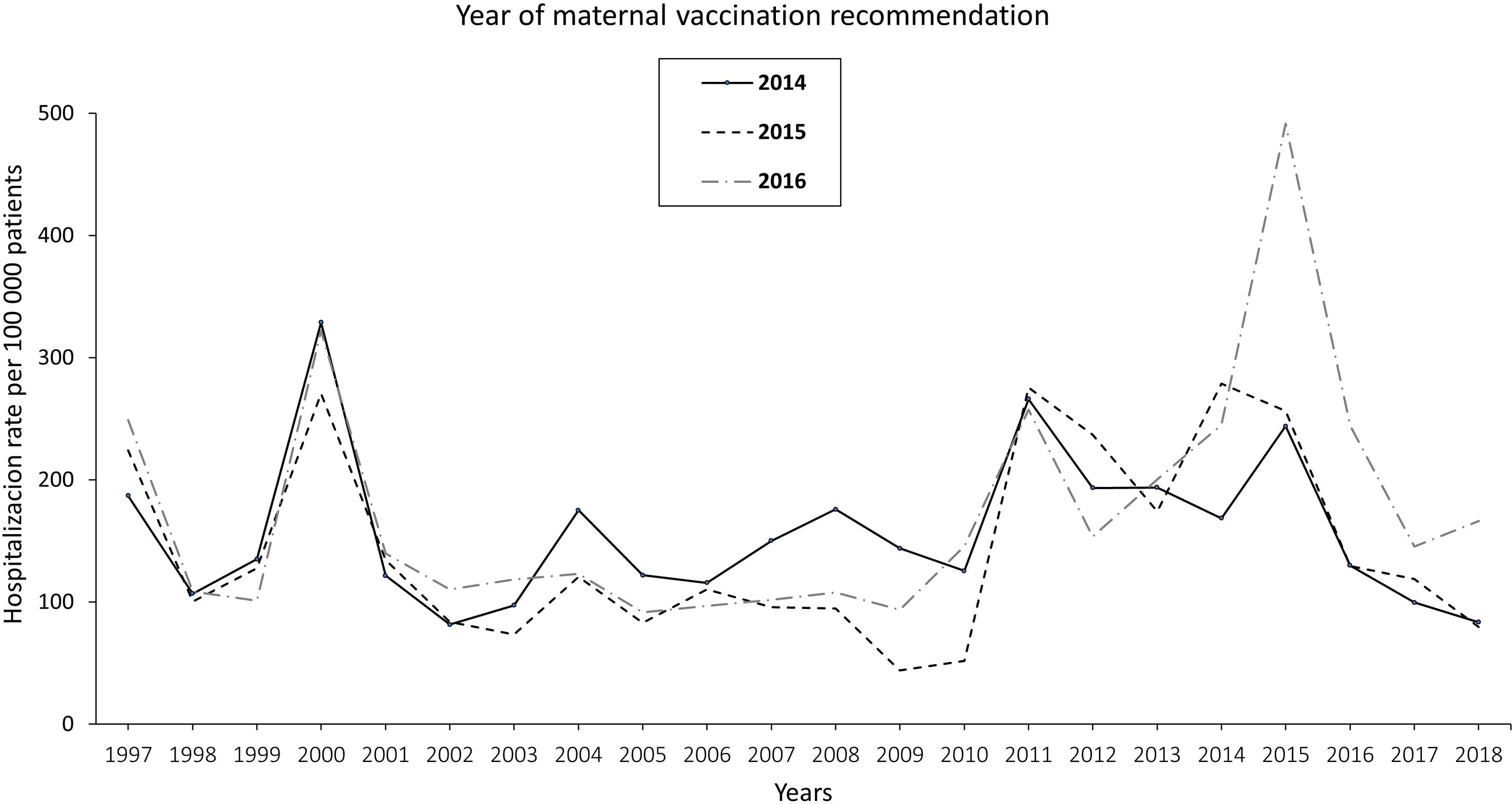
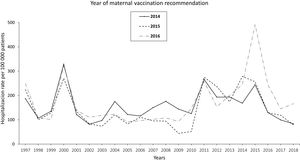
The pertussis hospitalization rate follows an increasing epidemic pattern similar to that of the IR in recent years, with the highest rate in children<1 year, especially in infants under 3 months.17 Vaccination has significantly reduced hospital admissions.18 The annual rate of hospitalizations in Spain per 100000 population in the period 1997–2010 was 131.02 cases in infants under 1 year, increasing to 250.13 in the period 2011–2015 and decreasing to 157.69 in 2016–2017. Most cases were recorded in infants ≤4 months, with hospitalization rates of 328.80, 670.81, and 385.84 cases/100000 population in the periods 1997–2010, 2011–2015, and 2016–2017, respectively. In total, 34, 36 and 4 deaths were reported in the three periods, respectively. Following the implementation of maternal vaccination by region, a 20% reduction in the rate of hospitalizations was observed19 (Fig. 1).
Annual rate of hospitalization associated with pertussis per 100000 children according to the year of introduction of maternal vaccination in Spain updated with 2018 data19.
The resurgence of pertussis has been attributed to several factors, with waning immunity and adaptation of the pathogen being particularly relevant.20 The introduction of acellular vaccines may have contributed to the emergence of new antigenic variants of B. pertussis capable of evading the vaccine-induced immune response, and strains that overproduce pertussis toxin (PT) or are deficient in pertactin (PRN), a protein essential for bacterial adhesion to the upper respiratory tract epithelium which is present in some vaccines.20,21 In Spain, antigen typing of B. pertussis strains isolated over the past 30 years has confirmed that after the transition from whole-cell to acellular vaccines, bacterial lineages that produce vaccine antigens different to those contained in current vaccines have emerged, as has been also confirmed in neighbouring countries.22,23
Microbiological diagnosisThe resurgence of pertussis highlights the need for rapid microbiological diagnosis that allows early implementation of adequate antibiotic therapy and the necessary measures to prevent bacterial transmission. This is also essential to identify changes in the disease due to modifications in vaccine effectiveness, waning immunity, the emergence of B. pertussis strains that can evade the immunity conferred by the vaccine, and to identify the emergence of other Bordetella species that cause clinical symptoms similar to those produced by B. pertussis, and for which the vaccines currently employed are not useful.24
Culture is the gold standard for the diagnosis of pertussis, although nucleic acid amplification techniques (NAATs) such as polymerase chain reaction (PCR) or isothermal amplification have become the most widely used diagnostic tools owing to their speed and excellent sensitivity.25 Moreover, NAATs do not depend on the bacterial viability, so bacteria can be detected even in cases of persistent cough lasting more than three weeks or after patients have been treated with antibiotics. In addition, most of the currently available NAATs can detect in the same test not only B. pertussis but also Bordetella species that cause pertussis-like disease, such as B. parapertussis, B. bronchiseptica or the recently described B. holmesii, which has been associated with major outbreaks and identified in 4.1% of laboratory-confirmed whooping cough cases between 2013 and 2016 in Barcelona.26 As macrolides show lower activity against B.holmesii than against other species, and that acellular vaccines do not confer cross-protection against this or other species such as B. parapertussis, accurate diagnosis of the pertussis-causing agent is needed in order to establish appropriate antimicrobial therapy, to determine the real incidence of pertussis and to assess the efficacy of the pertussis vaccines currently administered.27,28
Serological diagnosis is particularly indicated in advanced stages of the disease where both NAATs and culture offer less sensitivity. Titration of immunoglobulin Gs (IgGs) generated against PT provides better results, since the toxin is exclusively produced by B. pertussis and there is no cross-reactivity with similar antigens present in other microorganisms. However, serological diagnosis is not recommended during the year following vaccination, since the immune response induced by PT included in the vaccine cannot be differentiated from the response to natural infection.29,30
Antimicrobial resistanceMacrolides are the antibiotics recommended for both pertussis treatment and post-exposure chemoprophylaxis. Among them, the most widely used are azithromycin and clarithromycin, because they facilitate therapeutic adherence and eradicate the microorganism as effectively as erythromycin, with a high safety profile. In the case of neonates (under 1 month of age), azithromycin is the recommended antibiotic due to its good tolerance and high safety profile, while erythromycin has been associated with infantile hypertrophic pyloric stenosis.31
Over the past 20 years, B. pertussis strains that are resistant to macrolides have emerged as a result of mutations in their 23S ribosomal RNA encoding gene. Since the first description in the United States (US) in 1995, sporadic cases have been described in France, Iran and Japan.24,32,33 However, the high prevalence of macrolide-resistant B. pertussis detected in China since 2013 (>90%) is alarming.34,35 Accordingly, macrolide resistance in B. pertussis in Spain should be monitored to promptly identify any potential emergence and to establish the necessary measures to prevent its spread.
Prevention and current vaccine typesAfter the first vaccine authorized in the US in 1914, whole-cell pertussis (wP) vaccines combined with tetanus (T) and diphtheria (D) toxoids (DTwP vaccines) became available from 1948 onwards. Combined vaccines with inactivated poliovirus (IPV), Haemophilus influenzae type b (Hib) and hepatitis B (HepB) viral antigens were subsequently developed.
In Spain, inactivated acellular vaccines (DTaP) have been marketed since 1998, and have been progressively replacing DTwP vaccines due to their lower reactogenicity. Depending on the preparation, DTaPs may contain 1–5 B. pertussis antigens: PT (present in all), filamentous haemagglutinin (FHA), PRN, and one or two types of fimbrial proteins (FIM-2 and FIM-3). Multiple studies show that none of these factors alone is a determinant of vaccine efficacy, although most authors agree that the fundamental component is the PT that directly induces the generation of protective antibodies in response to immunization.36,37
DTaP vaccines: immunogenicity, efficacy, effectiveness and safetyDifferences among the populations analyzed, case definition criteria and the serological tests used make difficult to compare the immunogenicity and efficacy studies of the various DTaP vaccines. In Germany, only PT and PRN were found to be significantly associated with immune protection.38 Two other clinical efficacy trials in Sweden showed that after exposure to B. pertussis, the concentration of antibodies against the vaccine antigens was higher than that generated against other bacterial proteins not contained in the vaccine.39 Several clinical studies have estimated efficacies of about 84–85% and 71–78% in the prevention of typical and mild pertussis, respectively, for acellular vaccines formulated with at least three bacterial antigens.40,41 Simondon and colleagues estimated the efficacy of a 2-component vaccine at 85%, which led to its approval within the regulatory framework and implementation in vaccination programmes.42 A single-component vaccine (PT) is still in use in northern Europe, with an efficacy of 71%.43 However, the comparison of efficacy between wP and aP vaccines or between different aP vaccines has not led to clear conclusions, and there is no evidence to support the superiority of one aP vaccine over another.44,45
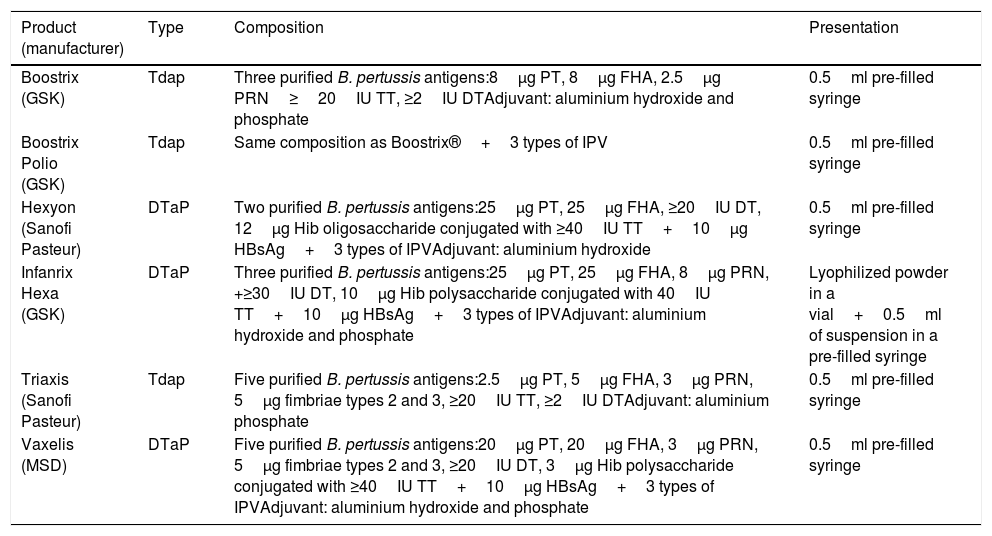
Most of the acellular vaccines currently available have shown their efficacy-effectiveness in various clinical trials and are currently used in national immunization programmes. Table 1 shows the acellular vaccines available and marketed in Spain.
Composition of pertussis vaccines commercially available in Spain.72
| Product (manufacturer) | Type | Composition | Presentation |
|---|---|---|---|
| Boostrix (GSK) | Tdap | Three purified B. pertussis antigens:8μg PT, 8μg FHA, 2.5μg PRN≥20IU TT, ≥2IU DTAdjuvant: aluminium hydroxide and phosphate | 0.5ml pre-filled syringe |
| Boostrix Polio (GSK) | Tdap | Same composition as Boostrix®+3 types of IPV | 0.5ml pre-filled syringe |
| Hexyon (Sanofi Pasteur) | DTaP | Two purified B. pertussis antigens:25μg PT, 25μg FHA, ≥20IU DT, 12μg Hib oligosaccharide conjugated with ≥40IU TT+10μg HBsAg+3 types of IPVAdjuvant: aluminium hydroxide | 0.5ml pre-filled syringe |
| Infanrix Hexa (GSK) | DTaP | Three purified B. pertussis antigens:25μg PT, 25μg FHA, 8μg PRN, +≥30IU DT, 10μg Hib polysaccharide conjugated with 40IU TT+10μg HBsAg+3 types of IPVAdjuvant: aluminium hydroxide and phosphate | Lyophilized powder in a vial+0.5ml of suspension in a pre-filled syringe |
| Triaxis (Sanofi Pasteur) | Tdap | Five purified B. pertussis antigens:2.5μg PT, 5μg FHA, 3μg PRN, 5μg fimbriae types 2 and 3, ≥20IU TT, ≥2IU DTAdjuvant: aluminium phosphate | 0.5ml pre-filled syringe |
| Vaxelis (MSD) | DTaP | Five purified B. pertussis antigens:20μg PT, 20μg FHA, 3μg PRN, 5μg fimbriae types 2 and 3, ≥20IU DT, 3μg Hib polysaccharide conjugated with ≥40IU TT+10μg HBsAg+3 types of IPVAdjuvant: aluminium hydroxide and phosphate | 0.5ml pre-filled syringe |
DT, diphtheria toxoid; FHA, filamentous haemagglutinin; HBsAg, hepatitis B surface antigen; Hib, Haemophilus influenza type b; IPV, inactivated poliomyelitis virus; PRN, pertactin; PT, pertussis toxoid; TT, tetanus toxoid.
In any case, the effectiveness of aP vaccines against pertussis is not optimal, and factors such as waning immunity over time and expansion of clones with antigenic polymorphism could explain why their effectiveness is the lowest within paediatric vaccines. Systemic and local adverse effects are generally less common with aP vaccines, although the rate and intensity of minor local adverse reactions tend to increase with each booster dose of DTaP.40,41,46 DTaP vaccines combined with other antigens (DTaP-IPV; DTaP-IPV/Hib; DTaP-IPV/Hib/HepB) have shown seroprotection and efficacy not inferior to non-combination vaccines and a safety profile comparable to that offered by separate administration of any of the components.47–51
Protection throughout life: new vaccination strategiesThe reduced effectiveness of acellular vaccines compared to other vaccines and the waning of immunity after the primary vaccination, inherent in both whole-cell and acellular vaccines underline the advisability of designing global prevention strategies that protect throughout life.6,52
Vaccination during pregnancyImmunization during pregnancy is currently considered the most effective and efficient vaccination strategy to protect infants from severe pertussis and associated mortality in the first months of life before they begin their primary vaccination course.53 This approach protects the newborn (NB) both directly by preventing infection and transmission from the mother, and indirectly by passive transplacental transfer of antibodies to the foetus, which will protect the baby until the first dose of vaccine is received at 2 months of age. There is a good correlation between maternal post-vaccine IgG1 concentrations and those detected in the umbilical cord, which has even higher levels. Thus, transplacental antibody transfer can protect the NB if the placenta is healthy.54
Early immunization during the second trimester of pregnancy has been associated with higher titres of transferred antibodies against PT and FHA than estimated when vaccination is given from gestational week 25.55 Anti-PT IgG avidity to PT in cord blood is higher in mothers vaccinated early in the third trimester than in those vaccinated later.56 However, a higher level of scientific evidence is still needed to determine the optimal time for immunization in pregnancy.
Maternal immunization with low-dose acellular pertussis vaccine (Tdap)57,58 has been shown to be safe, with no increased risk of maternal or foetal adverse effects (such as prematurity, premature rupture of membranes, intrauterine growth retardation, endometritis, or congenital malformations). In Spain, three acellular vaccines with a low antigenic load of pertussis component (Tdap) (Table 1) are indicated for protection not only during pregnancy, but also as a booster after primary immunization in children, adolescents and adults.
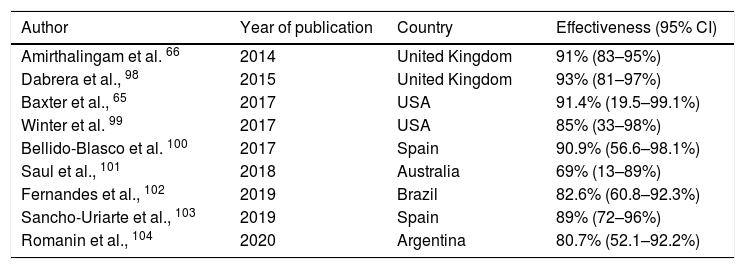
A small increase in the incidence of chorioamnionitis has been reported in vaccinated pregnant women, although this finding has not yet been corroborated by other authors.59 The main efficacy/effectiveness studies of vaccination in pregnancy for protection of the infant are shown in Table 2.
Main studies of the effectiveness of pertussis vaccination programmes in pregnant women.
| Author | Year of publication | Country | Effectiveness (95% CI) |
|---|---|---|---|
| Amirthalingam et al. 66 | 2014 | United Kingdom | 91% (83–95%) |
| Dabrera et al., 98 | 2015 | United Kingdom | 93% (81–97%) |
| Baxter et al., 65 | 2017 | USA | 91.4% (19.5–99.1%) |
| Winter et al. 99 | 2017 | USA | 85% (33–98%) |
| Bellido-Blasco et al. 100 | 2017 | Spain | 90.9% (56.6–98.1%) |
| Saul et al., 101 | 2018 | Australia | 69% (13–89%) |
| Fernandes et al., 102 | 2019 | Brazil | 82.6% (60.8–92.3%) |
| Sancho-Uriarte et al., 103 | 2019 | Spain | 89% (72–96%) |
| Romanin et al., 104 | 2020 | Argentina | 80.7% (52.1–92.2%) |
One aspect to consider is the potential interference of maternal antibodies with the infant's immune response to primary vaccination (immunological blunting), a phenomenon observed both after natural infection (e.g., measles, polio) and after vaccination (with significant variations between different vaccines and published papers).60 Although a lower rate of humoral response to the primary vaccination against pertussis has been described in infants of mothers vaccinated during pregnancy, this effect disappears after administration of the booster dose.61–63 The clinical relevance of these data is unknown, as countries with well-established maternal immunization programmes have not observed an increase in pertussis cases in infants or older children. A lower titre of anticapsular antibodies against some vaccine serotypes of Streptococcus pneumoniae has also been described, probably due to the effect of the non-toxic mutant of diphtheria toxin (CRM197) used as a carrier protein in the conjugated vaccines. Nevertheless, the percentages of seroprotection in infants of vaccinated and unvaccinated mothers were comparable after the primary vaccination and booster doses, except for serotype 3.64
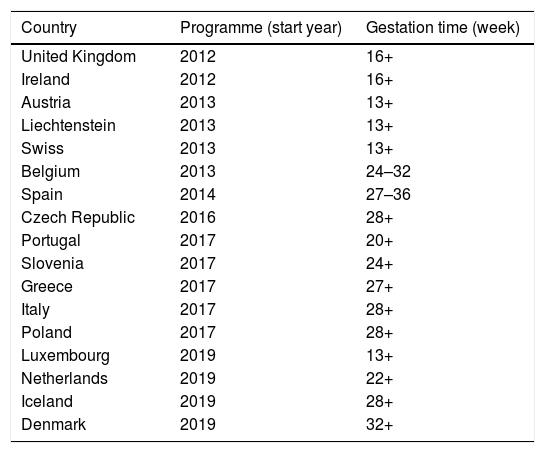
The US was the first country to introduce maternal vaccination in 2011, with a Tdap dose between weeks 27 and 36 of gestation and revaccination in every pregnancy. The estimated vaccine effectiveness in pertussis prevention during the first two months of life of infants was 91.4% (95% confidence interval [CI] 19.5–99.1%), and 69% (95% CI 43.6–82.9%) during the first 12 months of life.65 The United Kingdom (UK) incorporated it in 2012, reaching 64% coverage in the first year of implementation and 93% vaccine effectiveness in pertussis prevention during the first two months of life.66 According to 2019 data, maternal vaccination is already in place in 18 countries in Europe, although the gestational time at which it is administered differs (Table 3).
Recommendations for pertussis vaccination in pregnancy in Europe (data as of November 2019).
| Country | Programme (start year) | Gestation time (week) |
|---|---|---|
| United Kingdom | 2012 | 16+ |
| Ireland | 2012 | 16+ |
| Austria | 2013 | 13+ |
| Liechtenstein | 2013 | 13+ |
| Swiss | 2013 | 13+ |
| Belgium | 2013 | 24–32 |
| Spain | 2014 | 27–36 |
| Czech Republic | 2016 | 28+ |
| Portugal | 2017 | 20+ |
| Slovenia | 2017 | 24+ |
| Greece | 2017 | 27+ |
| Italy | 2017 | 28+ |
| Poland | 2017 | 28+ |
| Luxembourg | 2019 | 13+ |
| Netherlands | 2019 | 22+ |
| Iceland | 2019 | 28+ |
| Denmark | 2019 | 32+ |
+, starting from the week indicated.
In Spain, the Public Health Commission approved the anti-pertussis maternal vaccination programme in 2015,67 although it had already been implemented in some autonomous regions (like Catalonia and Asturias) since 2014. The vaccination schedule includes a dose of combined acellular vaccine with reduced diphtheria and pertussis toxoids content (Tdap) between weeks 27–28 of pregnancy; if there is risk of prematurity, the Spanish Association of Paediatrics (AEP) also recommends vaccination from week 16, preferably at week 20, after the scheduled 20-week ultrasound.68,69 The vaccine should be given in each pregnancy regardless of previous vaccination status. In Spain, current vaccination coverage in pregnant women is around 83%.70 The introduction of vaccination in pregnancy resulted in a reduction in the incidence of pertussis hospitalizations in infants, with significantly lower figures in the regions that adopted this strategy earlier19 (Fig. 1).
Primary vaccinationThe World Health Organization (WHO) recommends starting vaccination at 6–8 weeks of life and considers that it is essential not to delay its administration.71 The interval between primary vaccination doses should be ≥1 month, and the booster dose should be given at least 6 months after the second dose.
In Spain, pertussis vaccination began in 1975, with three doses of whole-cell vaccine before age 6–7 months. Doses at 15–18 months and 4–6 years were introduced in 1996 and 2001, respectively. Acellular vaccines subsequently replaced whole-cell vaccines, especially for the dose given at 18 months of age, due to their lower reactogenicity. Administration of a fifth dose at 4–6 years of age was incorporated into the vaccination schedule in 2005. In 2012, the second booster dose was replaced by the low-dose acellular vaccine (Tdap).
Since 2017, the Interterritorial Council of the Spanish National Health Service (CISNS) has recommended primary vaccination with hexavalent standard-dose vaccines (DTaP) at 2 and 4 months of age, and a booster dose at 11 months in a 2+1 schedule that remains in force today.69 To complete the vaccine schedule, a fourth dose should be given at 6 years of age with a combined vaccine (DTaP-IPV or Tdap-IPV). The fifth dose should be given at 12–14 years with tetanus and diphtheria vaccine, Td, although the Tdap vaccine has been administered in Asturias since 2017, as recommended by the AEP.68,72 Similar vaccine schedules, with slight variations in the number of doses and the time at which they are administered, are used in other European Union countries.73 Since 2020, the Centers for Disease Control (CDC) have authorized the use of Td or Tdap for tetanus, diphtheria, and pertussis vaccination in children>7 years old who are not vaccinated or who require booster doses.
Data are available on the impact of the administration of the first dose of pertussis vaccine in NB at 6 or 8 weeks, but few studies have compared immunogenicity and safety according to the time of administration.74 A phase III clinical trial of a monovalent acellular vaccine in NB confirmed satisfactory immunogenicity and safety, suggesting that it could be an alternative to protecting the child against pertussis in cases where the vaccine had not been administered during pregnancy.75 Preterm NBs (PNBs) have specific cellular immune responses. In the case of pertussis, no serological surrogate pattern of protection has been established. It has been observed that after the administration of combined vaccines with a 2+1 or 3+1 schedule, the IgG immune response of PNBs may be somewhat inferior to term NBs (TNBs). Even so, after the booster dose, antibody titres recover to similar levels. PNBs should complete the vaccination schedule based on their chronological age, following the same vaccination guidelines as TNBs.68,76,77 The hexavalent vaccines currently available in Spain can be used to vaccinate premature infants, although the potential risk of apnea must be evaluated and respiratory monitoring should be performed for 48–72h in very premature children (born at ≤28 weeks of gestation) and in particular, in infants with a history of respiratory immaturity.78
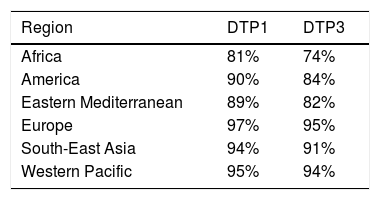
According to 2019 data, 90% of children received at least one dose of DTaP and 85% received three doses79 (Table 4). In Spain, in 2018, all autonomous regions except for the Balearic Islands (which did not provide information) reported coverage of more than 95% of the cohort for the first two doses of hexavalent vaccine during the first year. However, for the third dose at 11 months, six communities presented overall coverages slightly below 95.5%, with the lowest being in the Basque Country (92.5%).70
Vaccination coverage during routine immunization programmes with 1 or 3 doses of the DTP vaccine in the World Health Organization (WHO) regions in 2019.79
| Region | DTP1 | DTP3 |
|---|---|---|
| Africa | 81% | 74% |
| America | 90% | 84% |
| Eastern Mediterranean | 89% | 82% |
| Europe | 97% | 95% |
| South-East Asia | 94% | 91% |
| Western Pacific | 95% | 94% |
Despite the high coverage, the duration of the immunity conferred by primary vaccination (as with natural immunity after infection) is limited, a feature common to all types of vaccines (cellular and acellular).80 For each year after the last dose of acellular vaccine, there is an estimated 27–33% increase in the chances of developing pertussis, and that only 10% of infants vaccinated with standard-dose vaccines (DTaP) remain protected 8.5 years after the last dose.80,81 This justifies the need for booster doses at preschool, adolescent and adult age.
Preschool-age vaccine boosterVaccines and vaccine schedules used in primary vaccination can influence acquired immune protection. Consequently, preschool vaccination is essential for reducing the risk of pertussis in an environment where transmissibility conditions are very high. No significant differences have been found so far in the duration of immune protection acquired after a childhood vaccination series with 3 or 5 doses of DTaP.80 Nevertheless, published data in Australia showed that removing the booster dose at 18 months in 2003 resulted in an increase in pertussis notification rates in children aged 1–4 years, which translated to an increase in the prevalence of undetectable IgG levels from 1997 to 1998 to 2007.83 Continuous epidemiological surveillance is therefore essential to assess the impact of changes in vaccine guidelines on the incidence of pertussis in children.
The gradual decline in the protection acquired after the primary vaccination justifies the need for a booster in preschool age. It has been estimated that the odds of acquiring pertussis would increase almost linearly with age by an average of 42% for each year after the last dose a DTaP vaccine. The amount of protection depends on the effectiveness of the DTaP vaccine. An initial DTaP effectiveness of 95% would decrease to 71% after 5 years of last dose of vaccine and 90% effectiveness would decrease to 42% in the same period of time.84 In children aged 5–9 years, effectiveness would exceed approximately 75%, and more than 65% of children would remain immune to pertussis 5 years after the last dose of DTaP.85
Although low antigen load vaccines have demonstrated adequate antibody responses and lower reactogenicity than standard-dose preparations, the absence of studies evaluating the duration of acquired protection in preschool and school-age children ≥4 years prioritizes the use of DTaP vaccines in the booster dose. The estimated age for receiving this dose is 3–7 years. In Spain, the combined standard load vaccine (DTaP-IPV) is recommended whenever possible, and preferably the Tdap-IPV in the corresponding dose at 6 of age.69 Official agencies, such as the WHO and the CDC, also recommend the use of standard-dose preparations (DTaP-IPV) versus low-dose preparations (Tdap-IPV) for booster doses, as they offer greater immunogenicity and comparable reactogenicity.86,87
Vaccination of adolescents and adultsLoss of the immunogenicity acquired after vaccination makes adolescents and adults susceptible to re-infection, thus becoming potential sources of pertussis transmission to children and infants with whom they are in contact. The vaccination strategy for adolescents and adults has demonstrated its efficacy, safety and benefit.88,89 In the US, Tdap vaccination in adolescents has been effective in reducing the number of pertussis hospitalizations in infants.90 The AEP and agencies such as the CDC recommend vaccination with Tdap in adolescence (12–14 years).72,91,92 Currently, in Spain, Tdap vaccination at the age of 13 is only funded in Asturias. Most European Union countries include adolescent vaccination in their vaccine schedules.73
The recommendations of the Spanish Ministry of Health for adult vaccination are limited to pregnant women, health professionals working in paediatrics, obstetrics and their respective emergency areas, and professionals working in close contact with cases and in the control of pertussis epidemic outbreaks. These measures are supported by the AEP, which also proposes that childcare providers be included in the programme.93,94 In Spain, the approved vaccines for adolescents and adults are Boostrix and Triaxis (Table 1).
Vaccine boosters in adulthood can strengthen the level of immunization of the population and thus reduce bacterial circulation.6 The CDC has recently authorized the use of the Tdap vaccine in any adult situation, as a replacement or as a possible alternative to Td in cases where previously only Td was indicated for tetanus wound prophylaxis.95 In Spain, the current tetanus vaccination recommendation considers that the administration of 5 doses of Td throughout life is sufficient to protect against tetanus up to the age of 65, but this strategy fails to provide pertussis booster immunization in adults.96 In the European Union, several countries recommend a pertussis vaccine booster in adults every 10 years after completing the childhood immunization schedules.73
The proportion of older people is constantly increasing worldwide, especially in Europe, as is the risk of infectious diseases whose severity increases with age. Older people nowadays provide valuable family support by caring for infants and children, so this is a population group with a particular risk of infection and, consequently, of transmission to their setting. It would therefore be particularly advisable to consider extending the benefits of pertussis vaccination to this risk group.
Cocooning strategyThe cocooning strategy is based on the vaccination of individuals in the child's immediate environment (parents, grandparents, siblings, others who live with the child, etc.) to avoid disease transmission. Despite the potential difficulty implementing this strategy, it protects children whose mothers were not vaccinated during pregnancy. In countries such as Australia, France, Germany and the US, this measure was introduced in 2000. However, since the vaccine coverage achieved is suboptimal in most cases, this strategy is not sufficient in itself to prevent childhood infection and consequently, hospitalizations or deaths.71,97
Working Group recommendationsAll data presented reinforce the need for a comprehensive prevention strategy to protect all age groups susceptible to pertussis infection and transmission, that should include the following key points:
- •
Rapid, sensitive and specific microbiological diagnosis.
- •
Surveillance of the vaccine antigen variants of B. pertussis and susceptibility of B. pertussis to macrolides, as well as the emergence of other Bordetella species that can produce pertussis-like pathology.
- •
Vaccination in pregnancy with Tdap as a double measure of protection for mothers and infants under 2 months.
- •
Compliance with the current 2+1 primary vaccination schedule with hexavalent vaccines at 2 and 4 months of age, and booster at 11 months. Vaccination of PNBs (which can be done with hexavalent vaccines) must be completed according to the infants’ chronological age, following the same vaccination schedule as TNBs.
- •
Booster vaccination in preschool-age children as protection against the risk of infection associated with the loss of vaccine immunity achieved after the primary vaccination.
- •
Vaccination of adolescents to reduce the risk associated with loss of immunity.
- •
The implementation of complementary measures to the vaccination programme such as the cocooning strategy and the vaccination of adults and healthcare professionals.
The authors disclose receipt of the following financial support for the research, authorship, and publication of this article: Sanofi Pasteur (Madrid, Spain) supported this work but had no role in the preparation of the manuscript.
Conflict of interestsJuan José González-López has received compensation for consultant services for Sanofi Pasteur, has received research support from Sanofi Pasteur and GlaxoSmithKline (GSK) and has collaborated in educational activities funded by Sanofi Pasteur and Merck Sharp & Dohme (MSD). Javier Álvarez Aldeán has collaborated in educational activities funded by GSK, Pfizer, and Sanofi, has received compensation for services as consultant for Sanofi-Pasteur, GSK, MSD, and Pfizer and has participated as researcher in clinical trials funded by GSK, Novartis, and Sanofi Pasteur. Francisco José Álvarez García has collaborated in educational activities funded by GSK, MSD, Pfizer, and Sanofi Pasteur and has received compensation for services as consultant for Sanofi-Pasteur, GSK, MSD, and Pfizer. Magda Campins has participated as researcher in vaccine clinical trials funded by GSK, Novartis, Sanofi Pasteur and Novavax, and has received compensation for consultant services for Sanofi Pasteur, GSK, Pfizer, Novartis, and MSD. María Garcés-Sánchez has participated as researcher in vaccine clinical trials from GSK, Novartis, Sanofi Pasteur, and Pfizer, and has received compensation for consultant services for Sanofi Pasteur, GSK, Pfizer, and MSD. Ruth Gil-Prieto has received research grants from Merck, Sanofi Pasteur and Pfizer and has received compensation for services as consultant for Sanofi Pasteur. Ana Mª Grande-Tejada has collaborated in educational activities funded by GSK, MSD, Pfizer, and Sanofi Pasteur and has received compensation for services as consultant for Sanofi Pasteur GSK, MSD, Pfizer, and Novartis.
The authors thank Susana Cañón (Medical Statistics Consulting, S.L., Valencia, Spain) for providing medical writing support in accordance with Good Publication Practice (GPP3) guidelines, which was funded by Sanofi Pasteur (Madrid, Spain).