Introduction
Atherosclerosis is pathogenically similar to a chronic inflammatory response. Peripheral arterial occlusive disease (PAOD) is a common manifestation of atherosclerosis. PAOD is defined as obstructive arterial disease of the lower extremities that reduces arterial flow during exercise or, in advanced stages, at rest. PAOD represents a marker for premature cardiovascular events (eg, myocardial infarction, stroke) and vascular-related death 1.
Chlamydophila pneumoniae has been demonstrated to be a common cause of pneumonia, bronchitis, sinusitis, and pharyngitis both in adults 2 and children 3. Saikku and colleagues 4 showed an association between high titers of C. pneumoniae antibodies and coronary artery disease. In addition, an association between C. pneumoniae antibodies and carotid artery thickening has been reported 5. The presence of C. pneumoniae in atheroma of the coronary artery has been established in several populations by different diagnostic methods: in vitro cell culture (ICC), PCR, and electron microscopy (TEM) 6,7. The presence of C. pneumoniae was even demonstrated to be in macrophages and smooth muscle cells of atheromatous plaques of the aorta 8.
C. pneumoniae may thus have a role in the multifactorial pathogenesis of PAOD, a chronic inflammatory condition of the vascular wall 1,9,10. In the present study, we explored the presence of the organism in atheroma of the carotid artery and its susceptibility profile.
Methods
Twelve patients who were having carotid endarterectomy were enrolled in this study, but only nine samples arrived at the laboratory in optimal conditions. All had advanced atherosclerotic plaques.
The tissues studied were obtained from nine patients with PAOD during endarterectomy surgery between October 2001 and April 2002, at the Hospital Allende and Hospital Cordoba (Cordoba, Argentina). The local ethics committee approved the study protocol. Informed consent was obtained from all patients. The study complies with the Declaration of Helsinki.
The patients who were admitted to our emergency room because of acute chest pain were diagnosed as having unstable angina pectoris on the basis of coronary angiography, electrocardiography, clinical presentation, and laboratory parameters.
Immediately after collection, tissue specimens were classified macroscopically by the grade of damage, according to the following scale: grade 0, no visible lesion; grade1, fatty streak; grade 2, fibrolipid plaque; grade 3, advanced lesion with fibrosis, calcification, ulceration, or haemorrhage.
Sterile vials with sucrose-phosphate-glutamic acid (SPG) transport medium were sealed and opened only in the laboratory. Each carotid artery segment, of approximately 5 mm in length, was divided into three portions. One was conserved in a SPG media, for further ICC, the second was frozen and stored at 70 °C for PCR; and the last one was fixed in Karnofsky solution for TEM. Every assay included positive and negative controls, consisting in LLCMK2 cell cultures infected and mock-infected with a prototype strain of C. pneumoniae. The prototype strain of C. pneumoniae was the AR39 strain and was purchased from ATCC.
Isolation ofC. pneumoniae in cell culture
Cell line: LLCMK2 was obtained from Unidad de Estudios de Clamidias, Cátedra de Microbiología, Facultad de Farmacia y Bioquímica; UBA, Argentina. Host cells were grown in Eagle's minimal essential medium with non essential amino acids and 2mM L-glutamine (EMEM) (Gibco), and 10% fetal calf serum (Gibco), at 35 °C in 5% CO2.
For isolation of C. pneumoniae in LLCMK2 cell cultures tissue samples were placed in 3 ml SPG medium and homogenized on ice for 2 min in a sterile tissue grinder. The homogenate was cleared by centrifugation, inoculated to 4 cell monolayers in 96-well microtiter plates and centrifuged at 1800 x g at 35 °C for 60 min 11 and then supernants were replaced by chlamydial isolation medium consisting of EMEM with 1 ug of cycloheximide per ml (Sigma Chemical Co). Two blind passages were made after the first inoculation of each specimen. To find out whether samples were infected with C. pneumoniae, we carried out immunofluorescence assays (IFA) with monoclonal antibodies against Chlamydia lipopolysaccaride 12 and with monoclonal antibodies specific for C. pneumoniae (Dako). After 72h incubation the monolayers of each passage were fixed with ice-cold methanol. Isolation was considered positive if at least three cells of the infected monolayer showed the typical inclusions of C. pneumoniae. All 9 samples were inoculated by quadruplicate.
Molecular detection ofC. pneumoniae
A 437 base pair fragment corresponding to the C. pneumoniae PstI restriction fragment described by Campbell et al 13 was amplified by PCR, using primers HL-1 (5'-GTTGTTCATGAAGGCCTACT-3') and HR-1 (5'-TGCATAACCTACGGTGTGTT-3'). The previously described 13 PCR conditions were modified slightly as follows: clinical samples (0.2 ml of each homogenate) were pelleted by centrifugation (15,000 * g, 30 min) and incubated in proteinase K (200 μg/ml)-0.5% Tween at 55 °C for 60 min. Subsequently, the samples were boiled for 10 min. The PCR was carried out in 0.2 ml tubes containing PCR buffer (10 mM Tris [pH 8.5], 50 mM KCl, 0.1% Triton), 200 μM (each) deoxynucleoside triphosphate, 2.5 mM MgCl2, 0.5μM (each) primer, and 2 U of Taq DNA polymerase (Invitrogen, Brazil). Amplifications were carried out in a thermal cycle (Biometra, Göttingen, Germany). The conditions for PCR were as follows: reaction volumes were first heated for 2 min at 94 °C and then subjected to 35 amplification cycles of 1 min at 94 °C, 1 min at 55 °C, and 1 min at 72 °C. After the last cycle, samples were held for 7 min at 72 °C.
PCR products were visualized through 1.5% agarose gel stained with ethidium bromide.
The sensitivity of the reaction was analized by serial dilutions of a known bacterial suspension and it was determined to be of 1 IFU/reaction tube (not shown).
Negative samples were aliquoted and seeded with positive control [100 inclusion forming units (IFU)] of DNA and reamplified to rule out an inhibition of the PCR.
Transmission electron microscopy
For transmission electron microscopy, the arterial tissue was processed by a standard technique, sectioned, and stained with lead citrate and uranyl acetate. Each specimen was examined with a Jeol 1200 EX (Jeol, Tokio, Japan) transmission electron microscope. Ultra-thin sections were examined for bacterial structures; elementary and reticulate bodies (EB and RB) compatible with chlamydial organisms. Controls were processed in parallel with the samples from each patient 14.
Antibiotic susceptibility profiles
The antimicrobial agents roxithromycin (Aventis Pharma, Romainville, France), penicillin G (Sigma, USA), and azithromycin (Searle, Sintyal) were provided as powders and solubilized according to the instructions of the manufacturers. Susceptibility tests were performed on LLCMK2 monolayers grown in 96-well plates as described previously 15. Briefly, after the cell monolayer reached confluence, the growing medium was replaced by serum-free Eagle's minimal essential medium supplemented with 1 mg of cycloheximide per ml (Sigma, USA) and one of the studied antibiotics in twofold dilutions. Each well was inoculated with 0.1 μl of the test strain diluted to yield 10 3 inclusion-forming units (IFU/ml) per ml. After 72 h incubation the monolayers were fixed and an IFA was performed as stated before.
The MIC (minimal inhibitory concentration) was defined as the lowest concentration at which no IFU were observed. In accordance with established protocols 16, the minimal chlamydiacidal concentration (MCC) was subsequently determined by removing the drug-containing medium, washing the wells with phosphate-buffered saline, and adding fresh medium without antibiotics for further incubation for 72 h.
The cells were then disrupted with sterile glass beads and vigorous shaking for 2 h, harvested and inoculated on fresh monolayers, which were incubated for further 72 h, and stained as described above. The MCC was the lowest drug concentration that inhibited the production of IFU in the antibiotic-free passage. All experiments were made by quadruplicate.
Results
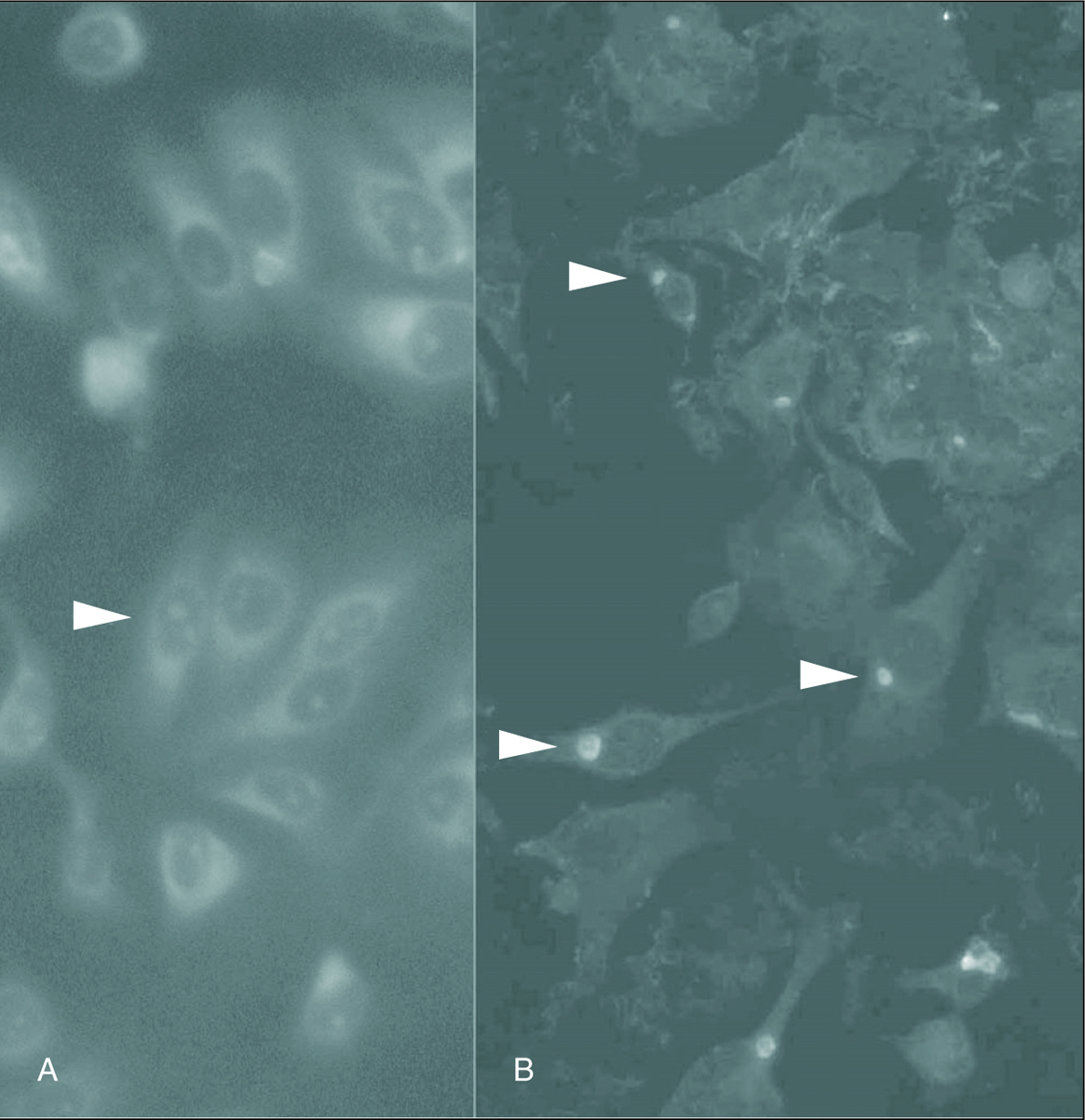
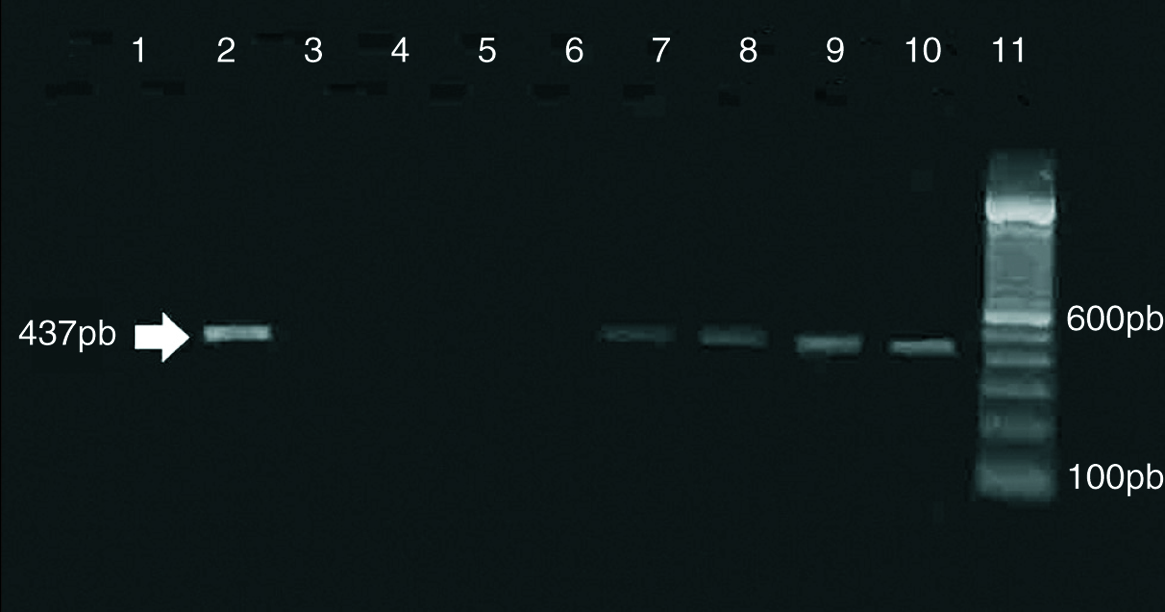
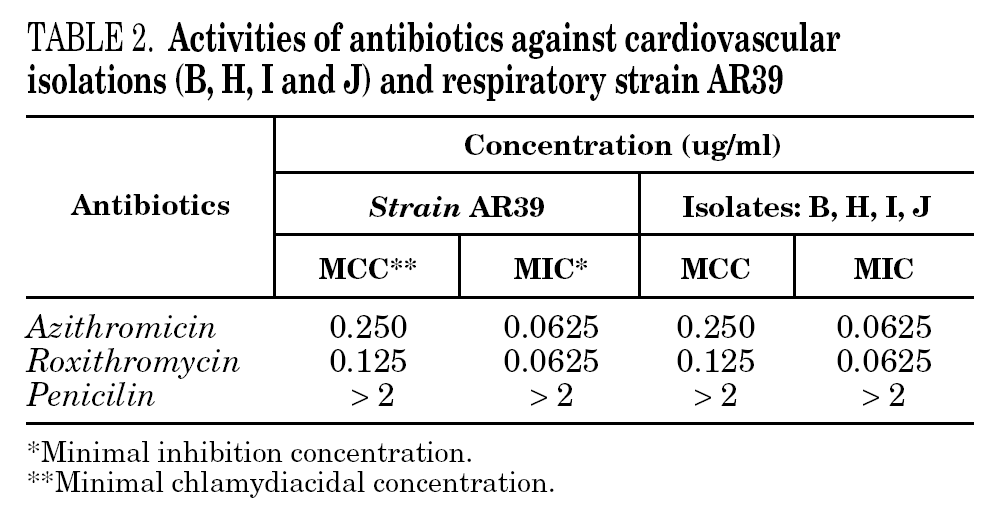
Inclusion-forming organisms which were IFA positive; were isolated from the carotid artery tissue samples B, H, I and J. Figure 1 shows photomicrographs of the ICC of sample B (fig. 1). PCR detection resulted equally sensitive for the four positive samples (fig. 2).
Figure 1. Detection of C. pneumoniae isolated from carotid artery atheromatous plaques in LLCMK2 cells, stained with fluoresceine-conjugated monoclonal antibodies specific for Chlamydia. A. Arrow indicates the LLCMK2 cells not infected. B. Arrow indicates the positive isolation. (Magnification: *400.)
Figure 2. Amplification of C. pneumoniae DNA from clinical specimens. Samples were amplified by using HL-1-HR1 primer set. Lanes 1, 3, 4, 5, 6: specimens isolation negative for (A, C, D, F, G). Lanes 2, 7, 8, 9: specimens isolation positive for (B, H, I, J). Lane 10: specimen isolation positive for C. pneumoniae AR39. Lane 11: molecular weight markers.
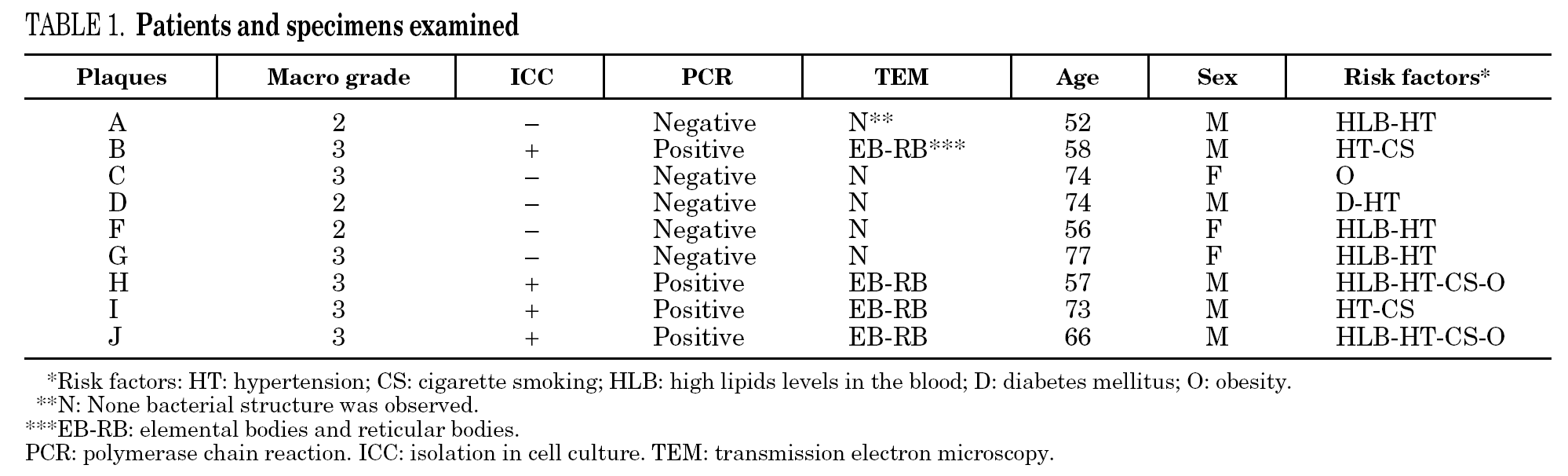
We were able to detect amplification of strains B, H, I and J during the first 4 passages. The results are shown in table 1.
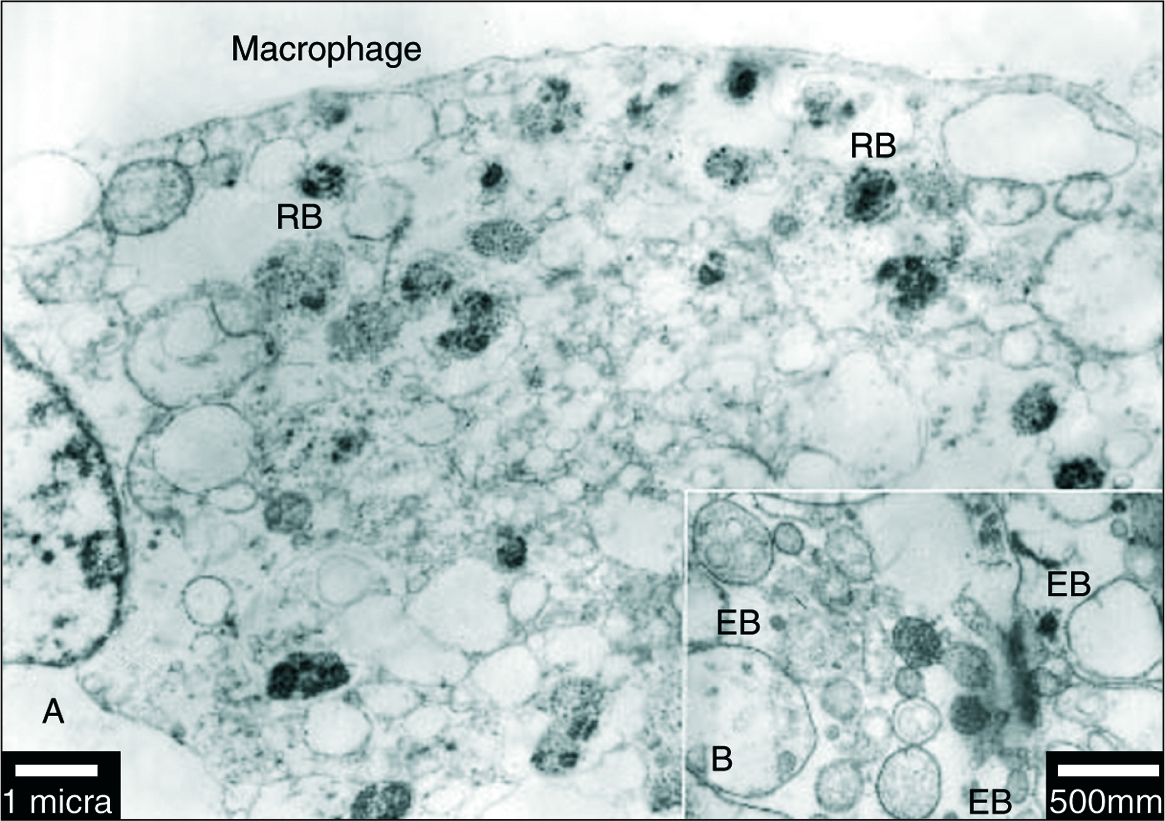
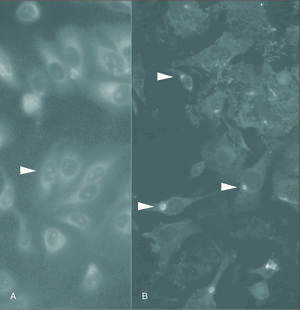
Stuctures similar in morphology and size to those of elementary (EB) and reticular bodies (RB) of C. pneumoniae were detected in four of the specimens when analyzed by TEM (fig. 3B). The EB were detected as particles with a diameter of 100 to 300 nm, surrounded by a membrane, and containing an electron-dense core measuring up to 100 nm in diameter (fig. 3B). The EB and RB were also identified in macrophages cells (fig. 3A).
Figure 3. A) Transmission electron micrograph of a macrophages cell in an area of atheroma within an artery. The macrophages is identified as a cell full of lipid droplets and vacuoles containing C. pneumoniae (RB). The scale bar represents 1 mm. B) Transmission electron micrograph of an atheromatous arterial wall. Elementary bodies of C. pneumoniae appear pear-shaped in some profiles (EB) and contain a central, electron-dense core. The scale bar represents 500 nm.
The four samples positives for C. pneumoniae were classified as grade 3 lesions.
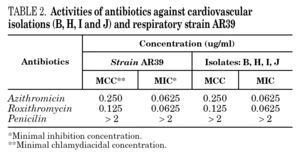
The four isolations positive from carotid lesions B, H, I, J and respiratory reference strain C. pneumoniae AR39 were compared for antibiotic susceptibility profiles. The results of MIC and MCC tests are summarized in table 2.
All MICs and MCCs values were within the range known for respiratory isolates 16, and all strains presented similar sensitivity profile to the antibiotics tested.
Discussion
The first study that indicated a possible association between C. pneumoniae and coronary artery disease was performed in Finland and showed that patients with coronary artery disease were significantly more likely to have serologic evidence of past infection with C. pneumoniae than controls 4. Since that time, serologic studies from the United States and other countries have demonstrated similar findings among patients with coronary artery disease 17,18 and in patients with thickening of the carotid arteries as well 19.
The important observations in this study are as follows: the detection of viable C. pneumoniae in 4/9 of arterial atheromatous lesions, in keeping with the results of some previous studies in which the organism has been detected in 71 to 100% of lesions 14,19. C. pneumoniae was detected in atherosclerotic plaques for all (44%) smoking patients with PAOD but was not detected in nonsmokers. There is evidence for a strong association between chronic infection with C. pneumoniae and smoking. It is concluded from the present data that chronic infection with the pathogen would not be an independent risk factor for carotid atheromatous lesions.
This study provides direct evidence to show the presence of viable C. pneumoniae in the carotid atheroma of patients with PAOD of Córdoba, Argentina. C. pneumoniae is a pathogenic organism which has been shown in vitro to be capable of infecting aortic smooth muscle cells, endothelial cells, and macrophages 20, all of which are involved in atherogenesis 21. The finding of the organism in the arterial lesion does not prove that C. pneumoniae is the leading cause in the progression of atherosclerosis. However, having a known pathogen replicating in the atheroma cells suggests that C. pneumoniae might be involved in the pathogenesis of PAOD.
Systemic dissemination of C. pneumoniae from the respiratory tract to the cells of the vascular wall requires a cellular transport system. Circulating monocytes, which are pivotal to the development of atherosclerosis, are known to migrate into the vascular wall 21. The finding of C. pneumoniae in macrophage cells raises the possibility that the bacteria reach the vascular wall through the infection of monocytes.
Numerous electron microscopy studies have more finely characterized the morphology of EB and RB, the continuum of intermediate forms, and the inclusion membrane within which the entire growth phase of the development cycle takes place. In this study we observed structures similar to inclusions of C. pneumoniae and typical elementary bodies (pear-shaped) in the macrophage cells of original specimens from atheromatous plaque. Extensive work is needed to determine if the bacteria can complete an infective cycle in these cells.
This is the first study that describes the antibiotic susceptibility profile of peripheral arterial strains in Argentina. It is important to note that antibiotics have been demonstrated to be active in vitro against C. pneumoniae. Thus, chlamydial eradication from cells other than monocytes/macrophages is potentially feasible. However, there is evidence suggesting that persistence may be established in those cells, too 22.
The results of trials investigating a possible effect of antibiotics on coronary artery disease are contradictory 23-29. Negative outcomes might be explained not only by the use of an incorrect hypothesis (that infection is not atherogenic) but also by an inadequate sample size or by the use of an ineffective antibiotic regimen.
Non-selective use of roxithromycin is inadequate for prevention of restenosis after coronary stenting. There is, however, a differential effect dependent on C. pneumoniae titres. In patients with high titres, roxithromycin reduced the rate of restenosis 30.
A possible role of C. pneumoniae in peripheral arterial occlusive disease (PAOD) is intriguing but has rarely been investigated. A randomized trial of roxithromycin versus placebo was carried out with a subgroup of the patients. Antibiotic medication showed effective in inhibiting progression of PAOD 31.
A beneficial effect of 4-week roxithromycin treatment on PAOD was observed during a follow-up period of 2.7 years 32. In that study 32, C. pneumoniae seropositivity had been associated with PAOD but not with coronary artery disease or cerebrovascular occlusive disease. A preference of C. pneumoniae for peripheral vessels is conceivable, since lower limb arteries have a different embryological origin and different histological constitution than coronary and cerebral arteries 33. That study provides strong evidence that C. pneumoniae is involved in the progression of PAOD and that antibiotic treatment directed against C. pneumoniae is effective in inhibiting this process.
Although C. pneumoniae infection can be effectively treated with tetracycline and macrolide drugs, it is not known whether the organisms in atheroma; could be eradicated by treatment or whether eradication of the organisms would have a favorable effect on the lesions. A controlled treatment trial with an effective antibiotic might be considered for future investigations. In our study, no difference was observed among the vascular isolates or between the isolates and the respiratory strains previously described. Apparently, vascular isolates of C. pneumoniae would not have a distinctive susceptibility profile. This confirms the finding that the C. pneumoniae isolates obtained so far are very homogeneous in their genetic background, protein profile, and immunogenicity. However, since a genetic typing system has not yet been established for C. pneumoniae, the presence of a genotype with a distinct vascular pathogenicity cannot be excluded.
Our results emphasize the potential role of C. pneumoniae in the etiology of atherosclerosis and suggest that the development of atheroma could be prevented by antibiotic treatment in patients in patients with proved C. pneumoniae infection.
Acknowledgments
This study was supported by a grant from the Fundación Roemmers Argentina. We would like to thank Claudia Nome for excellent technical assistance.
Correspondencia: C. Cuffini. PhD.
Fleming, s/n. Villa Santa Cruz del Lago. Córdoba 5152. Argentina.
Correo electrónico: ceciliacuffini@hotmail.com and ccuffini@cmefcm.uncor.edu
Manuscrito recibido el 29-3-2005; aceptado el 1-6-2005.