Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG) infections are a public health problem, worsened by frequent reinfections, whose incidence rate is not known in Spain. The objective of this study is to estimate in patients diagnosed with NG, CT or mixed infection (NG and CT): (1) the incidence of reinfections by the same microorganism, (2) the total incidence of Sexually Transmitted Infections (STI), both by the same microorganism and by infections other than the initial one, and (3) to identify predictors of reinfection.
MethodsObservational prospective case series involving 986 patients with CT and/or NG at specialized STI clinics in Biscay (Spain) between 2016 and 2019.
ResultsThe six month cumulative incidence of reinfection by the same microorganism was 17.24% (CI95%: 14.9–19.7) and 24.65% (CI95%: 21.9–27.4) for any STI (reinfection or other). Being an immigrant (OR=1.8; CI95%: 1.3–2.6), men who have sex with men (OR=1.8; CI95%: 1.3–2.6), number of sexual partners (OR=4.3; CI95%: 2.7–6.8 for more than 5 partners), having a new partner (OR=1.7; CI95%: 1.08–2.6), not always using a condom (OR=1.4; CI95%: 1.02–1.9) and consumption of alcohol prior to sex (OR=3.8; CI95%: 1.5–9.5) were associated with reinfection by any STI.
ConclusionThese characteristics allow doctors to identify patients in whom to prioritize short-term rescreening for repeated infections with any STIs after initial treatment for NG or CT.
Las infecciones por Chlamydia trachomatis (CT) y Neisseria gonorrhoeae (NG) son un problema de salud pública, agravado por frecuentes reinfecciones, cuya incidencia desconocemos en España.
ObjetivosEstimar en pacientes diagnosticados de NG, CT o infección mixta (NG y CT): 1) la incidencia de reinfecciones por el mismo germen, 2) la incidencia total de infecciones de transmisión sexual (ITS), tanto por el mismo germen, como por infecciones diferentes a la inicial y 3) identificar características que predicen la reinfección.
MétodosEstudio observacional prospectivo de una serie de casos: 986 pacientes diagnosticados de CT y/o NG en las consultas de ITS de Bizkaia (España) entre septiembre de 2016 a enero de 2019.
ResultadosEn 6 meses de seguimiento promedio la incidencia de reinfección por el mismo germen fue del 17,24% (IC95%: 14,9-19,7) y la de cualquier ITS (reinfección u otra) del 24,65% (IC95%: 21,9-27,4). Los factores asociados con la reinfección por cualquier ITS fueron: ser inmigrante (OR=1,8; IC95%: 1,3-2,6), hombre que tiene sexo con hombres (OR=1,8; IC95%: 1,3-2,6), número de parejas sexuales (OR=4,3; IC95%: 2,7-6,8 para más de 5 parejas), tener una pareja nueva (OR=1,7; IC95%: 1,08-2,6), no utilizar siempre preservativo (OR=1,4; IC95%: 1,02-1,9) y consumo de alcohol en relación al sexo (OR=3,8; IC95%: 1,5-9,5).
ConclusiónEstas características sirven para identificar pacientes de alto riesgo en los que priorizar el rescreening de ITS tras una infección, que debe ser completo, incluyendo otras infecciones diferentes a la inicial.
Their growing incidence and consequences on reproductive health make Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG) infections an important public health problem,1,2 worsened by frequent reinfections,3,4 which cause more severe complications and increase the risk of HIV infection.5,6 For this reason, in different countries rescreening for these infections after their treatment is recommended,7–10 but these recommendations differ with respect to the selection of the candidates to be screened, the time interval and the need to test for both infections or only for CT.
The incidence of gonorrhoea in Spain has multiplied in the last 15 years, from 2.9 in 2005 to 28.9/100,000 in 2019.11 That of CT (44.2/100,000 in 2019) is lower than in the European Union (146/100,000),11,12 possibly due to underdiagnosis and underreporting (its declaration to the Spanish National Epidemiological Surveillance Network is not implemented in the entire country). Regarding NG and CT reinfections, there are few studies in our country. A retrospective study of patients treated in a clinic for Sexually Transmitted Infections (STI) between 2007 and 2015 estimated a 18% of CT reinfections,13 and López-Corbeto et al. a 10.3% in women under 25 years.14 As far as we know, there are no studies of reinfection by NG.
In addition to the scarcity of epidemiological information and perhaps as a consequence of it, clinical practice for STIs in Spain varies greatly, from episodic and sometimes empirical treatment in primary care, emergency, gynaecological, urological, and dermatological services, to its comprehensive management and control in specialized STI clinics. Without national studies, rescreening for these infections is carried out based on international recommendations only in some STI centres. It is imperative, therefore, to know the frequency and epidemiology of the reinfections to assess rescreening necessity and to determine how to do it.
The objectives of our study are: (1) to estimate the incidence of reinfections by the same microorganism among patients diagnosed with NG, CT or mixed (NG and CT) infections; (2) to estimate the total re-incidence of STIs in these patients, including both reinfections by the same microorganism and STIs other than the initial one; and (3) to identify the socio-demographic and behavioural characteristics that predict reinfection, in order to identify high risk groups in whom rescreening is more beneficial.
Materials and methodsAn observational prospective case series study was carried out between September 2016 and January 2019, involving all patients diagnosed with CT and/or NG infection in specialized STI clinics of the Infectious Diseases and Microbiology Services of the public Bilbao-Basurto Integrated Care Organization (Basque Health Service). These clinics serve the whole population of Biscay (Spain) (1,152,651 inhabitants). The study was approved by the Clinical Research Ethics Committee of the Basque Country and all the participants signed an informed consent in order to be included.
Samples were collected from all the patients (symptomatic or asymptomatic) in order to detect NG and CT from all the locations susceptible to infection. For the microbiological study, cultures as well as molecular biology techniques were used. For the NG culture, GC-Lect plate was used (BD GC-Lect Agar, Becton Dickinson, Heidelberg/Germany). The molecular biology techniques were performed with the BD MAX CTGC TV2 (Becton Dickinson, Heidelberg/Germany) amplification technique of nucleic acids that simultaneously detects NG, CT and Trichomonas vaginalis in urine, endocervical, urethral, pharyngeal and rectal samples sent by means of a universal transport medium (UTM) (Copan).
The inclusion criterion was having an isolation of NG or CT, the exclusion criterion being a transient person and/or a language barrier that made it difficult to understand the informed consent.
Treatments followed clinical practice guidelines.8,15 All the patients were informed of the need to abstain from sex for a week from the start of treatment and until a week after sexual contacts had been treated and resolution of their symptoms, as well as the reasons for studying their sexual contacts, providing them with an appointment. All of them had a control visit one month after the treatment in order to confirm resolution of symptoms, compliance with therapy and abstinence from sex during the specified time and ensure partner notification. In gonococcal infection (GI), a test of cure was always carried out as well as in the CT infections in case of persistence of symptoms, suspicion of re-exposure, poor adherence to treatment, pregnancy and rectal chlamydia treated with azithromycin.7,8 Appointments for all patients were made four months after this control visit in order to carry out a complete STI rescreening.
The reinfection was defined as a positive test of CT or NG if more than 4 weeks had passed since treatment and the adherence to it had been correct. If the patient did not attend an agreed appointment, (for control or re-screening) they were contacted by phone to rearrange an appointment. A patient was considered lost after non-appearance for at least 2 newly programmed appointments, he/she was impossible to contact, or said that he/she did not wish to return.
AnalysisDescriptive measures of central tendency and dispersion for quantitative variables and proportions for categorical variables were calculated to summarize data, which were compared between subgroups using Student's t and chi-squared tests.
The accumulated incidence was calculated with their confidence intervals at 95% (CI95%) using the exact binomial distribution. In order to identify factors associated with a higher incidence, univariate and multivariate logistic regression analyses were performed including the following variables: type of initial infection (NG, CT or mixed), gender, age, country of origin, sexual preference, compliance with the partner notification, HIV infection, history of STIs, prostitution, pay for sex, and since treatment of the initial infection: abstention from sex for a week from the start of treatment, number of partners, steady partner, new partner, drug and/or alcohol use and condom use. The association measurement used was the odds ratio (OR) and its Wald CI95% was estimated. The analyses were made with SAS version 9.4 (SAS Institute, Cary, NC, USA), following backward and forward strategies to simplify the statistical models, using type III likelihood ratio tests for selecting variables. The level of statistical significance was 0.05 for all the statistical tests.
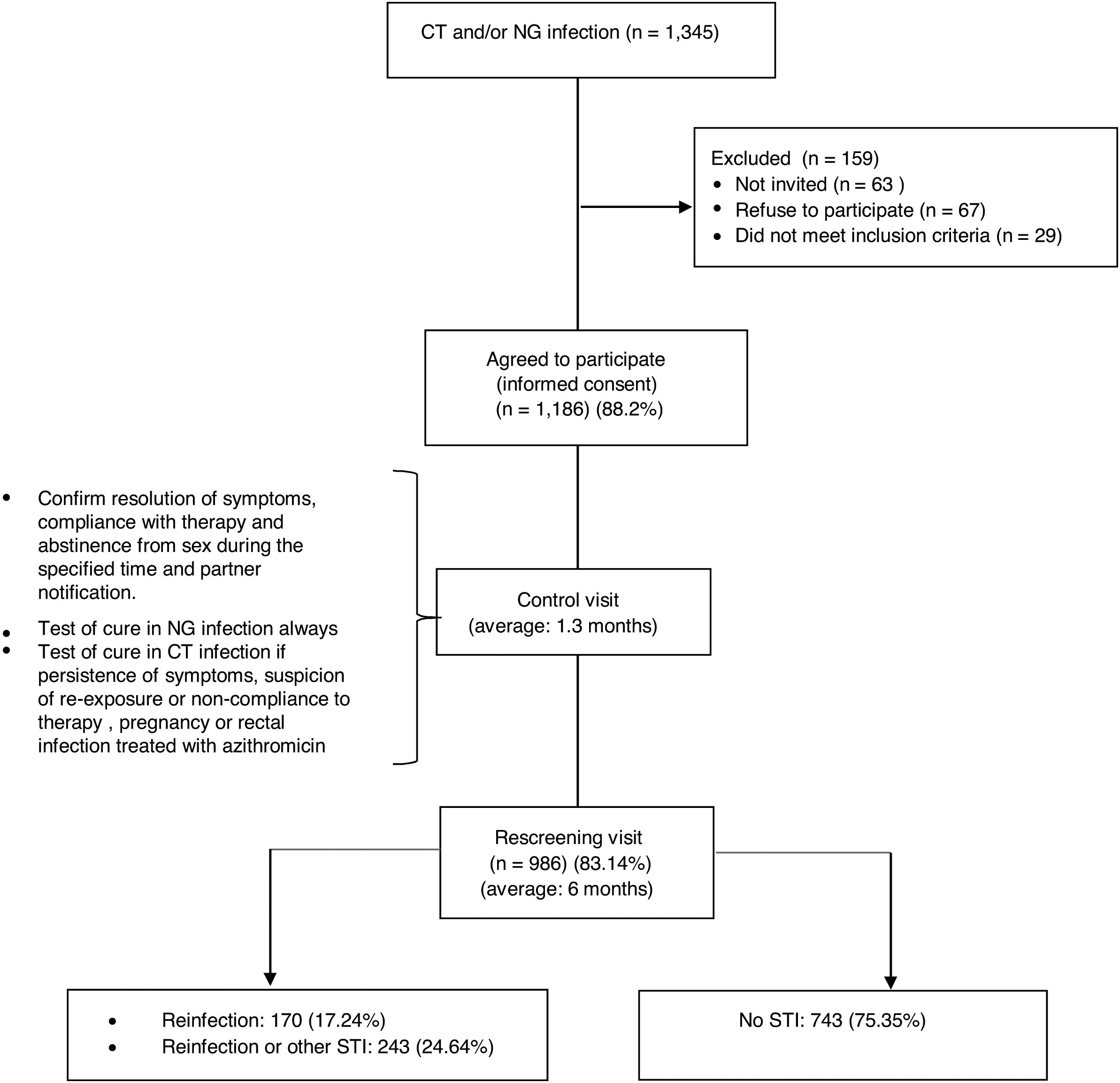
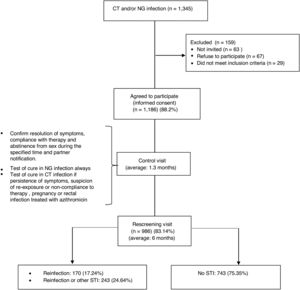
ResultsDuring the 29 month period of inclusion of participants in the study 1345 patients were diagnosed with NG and/or CT infections, of which 67 (4.97%) refused to participate and 63 (4.68%) did not return to the consultation. Of the total, 29 were excluded (2.14%) due to being transient or language barrier. No differences were found with respect to gender, age or country of origin between the 1186 that accepted (88.2%) and those that refused or could not be invited to participate. The study ended with 986 patients (83.14%), those that did not complete it were younger than completers (average age of 29.7 years vs. 34, p<0.001) and in a greater proportion, heterosexuals (HTX) (20.13% vs. 10.25% p<0.001) (Fig. 1).
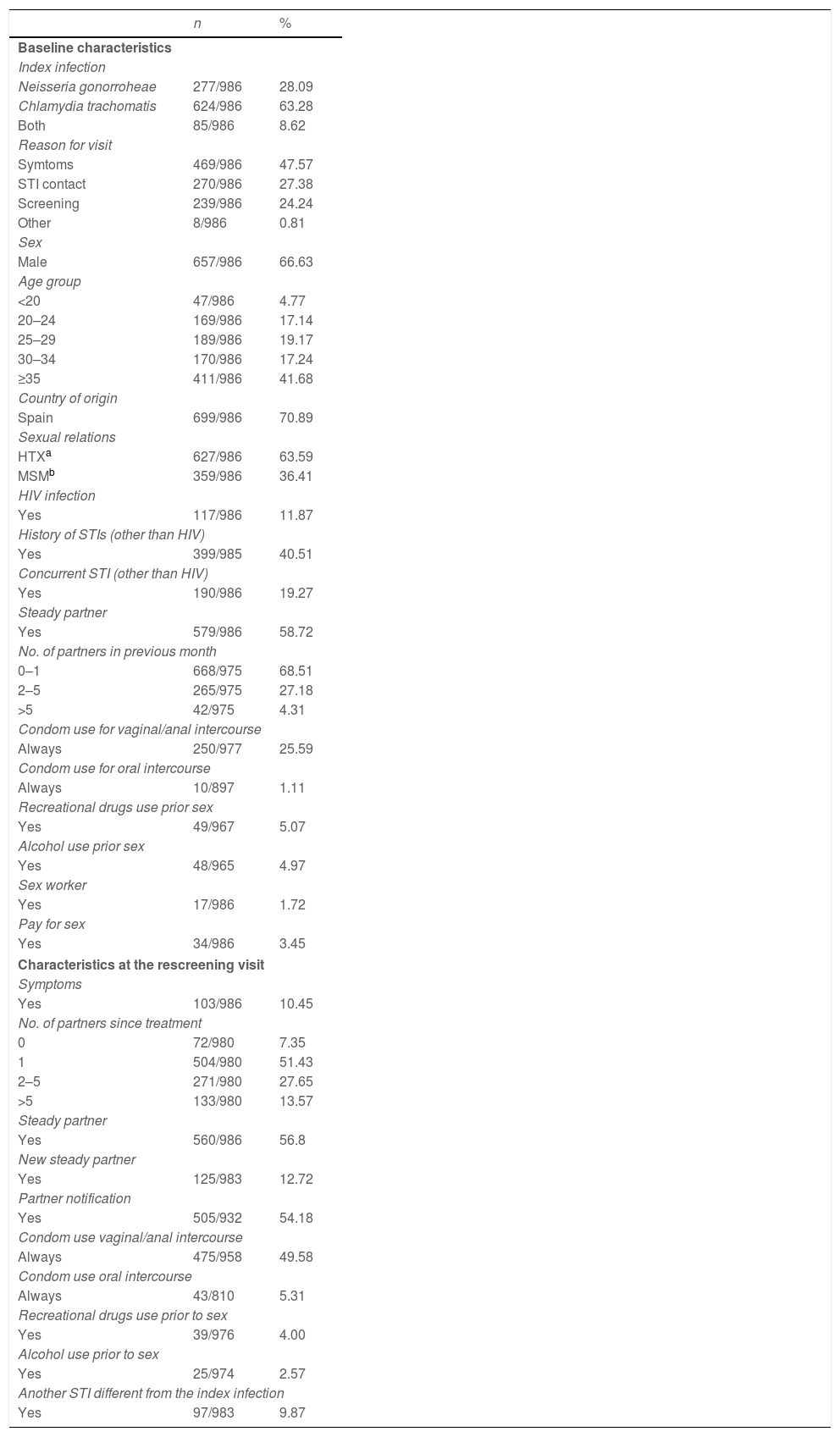
Characteristics of the participants who completed the studyTwo hundred seventy seven (277) patients were diagnosed upon entry in the study with GI, 624 with a CT infection and 85 with both. 66.6% were male and the average age was 34 years (range 14–72), higher among men (35.2 vs. 31.6 in women, p<0.0001). 29% were immigrants and 359 (36.4%) men who had sex with men (MSM). Of the total, 117 (11.8%) had HIV co-infection (95% of them MSM). Three hundred ninety eight (398, 40.36%) patients, had prior history of STIs, 69% of the MSM and 24% of the heterosexuals (p<0.0001). One hundred ninety (190, 19.3%) patients presented other STIs simultaneously (27.6% of the MSM and 14.5% of the heterosexuals, p<0.0001): syphilis (6.2%), condylomas (5%), genital herpes (4.5%), new HIV (1.3%) and trichomoniasis (1%). One out of every four participants reported that they always used condoms in vaginal/anal sex while only 1% always used them for oral sex. The use of alcohol or drugs prior to sex was reported by 5%, with a higher use of drugs among the MSM (11.3% vs. 1.61%, p<0.0001) (Table 1).
Characteristics of the 986 participants who completed the study.
| n | % | |
|---|---|---|
| Baseline characteristics | ||
| Index infection | ||
| Neisseria gonorroheae | 277/986 | 28.09 |
| Chlamydia trachomatis | 624/986 | 63.28 |
| Both | 85/986 | 8.62 |
| Reason for visit | ||
| Symtoms | 469/986 | 47.57 |
| STI contact | 270/986 | 27.38 |
| Screening | 239/986 | 24.24 |
| Other | 8/986 | 0.81 |
| Sex | ||
| Male | 657/986 | 66.63 |
| Age group | ||
| <20 | 47/986 | 4.77 |
| 20–24 | 169/986 | 17.14 |
| 25–29 | 189/986 | 19.17 |
| 30–34 | 170/986 | 17.24 |
| ≥35 | 411/986 | 41.68 |
| Country of origin | ||
| Spain | 699/986 | 70.89 |
| Sexual relations | ||
| HTXa | 627/986 | 63.59 |
| MSMb | 359/986 | 36.41 |
| HIV infection | ||
| Yes | 117/986 | 11.87 |
| History of STIs (other than HIV) | ||
| Yes | 399/985 | 40.51 |
| Concurrent STI (other than HIV) | ||
| Yes | 190/986 | 19.27 |
| Steady partner | ||
| Yes | 579/986 | 58.72 |
| No. of partners in previous month | ||
| 0–1 | 668/975 | 68.51 |
| 2–5 | 265/975 | 27.18 |
| >5 | 42/975 | 4.31 |
| Condom use for vaginal/anal intercourse | ||
| Always | 250/977 | 25.59 |
| Condom use for oral intercourse | ||
| Always | 10/897 | 1.11 |
| Recreational drugs use prior sex | ||
| Yes | 49/967 | 5.07 |
| Alcohol use prior sex | ||
| Yes | 48/965 | 4.97 |
| Sex worker | ||
| Yes | 17/986 | 1.72 |
| Pay for sex | ||
| Yes | 34/986 | 3.45 |
| Characteristics at the rescreening visit | ||
| Symptoms | ||
| Yes | 103/986 | 10.45 |
| No. of partners since treatment | ||
| 0 | 72/980 | 7.35 |
| 1 | 504/980 | 51.43 |
| 2–5 | 271/980 | 27.65 |
| >5 | 133/980 | 13.57 |
| Steady partner | ||
| Yes | 560/986 | 56.8 |
| New steady partner | ||
| Yes | 125/983 | 12.72 |
| Partner notification | ||
| Yes | 505/932 | 54.18 |
| Condom use vaginal/anal intercourse | ||
| Always | 475/958 | 49.58 |
| Condom use oral intercourse | ||
| Always | 43/810 | 5.31 |
| Recreational drugs use prior to sex | ||
| Yes | 39/976 | 4.00 |
| Alcohol use prior to sex | ||
| Yes | 25/974 | 2.57 |
| Another STI different from the index infection | ||
| Yes | 97/983 | 9.87 |
The average time between the initial visit and the follow-up visit was 42 days (median 39), 99.3% had completed treatment and 6.3% reported having had sex within 7 days after treatment.
Median follow-up between the initial visit and the re-screening was 5.7 months, ranging from 3.5 to 9.5 months in 90% of the participants.
Of the total, 89.5% were asymptomatic when they returned to the rescreening visit, their average number of sexual partners since the treatment was 3.7:6.5 among MSM vs. 2.2 in the heterosexuals (p<0.0001); 560 (56.8%) stated having a steady partner and nearly 13% a new partner subsequent to the treatment. Partner notification was done in 54.2% of the participants. Certain changes in behaviour were detected between the initial visit and the rescreening: the percentage of those who reported always using a condom in vaginal/anal sex increased from 25.6% to 49.6%, 27% of participants (CI95%: 24.05–29.98) who initially reported never or occasionally using them went on to use them consistently (30.4% among HTX vs 20.8% among MSM, p=0.0019); for oral sex, 4.3% (CI95%: 2.90–5.81) of those who initially reported never or occasionally using them, went on to use them consistently, without differences between HTX and MSM; the reported consumption of alcohol associated with sex decreased from 5% to 2.5% with respect to the initial visit and the consumption of toxic substances was more frequent among MSM (5.1% vs. 1.13% in alcohol and 10.4% vs. 0.32% in drugs, p<0.001) (Table 1).
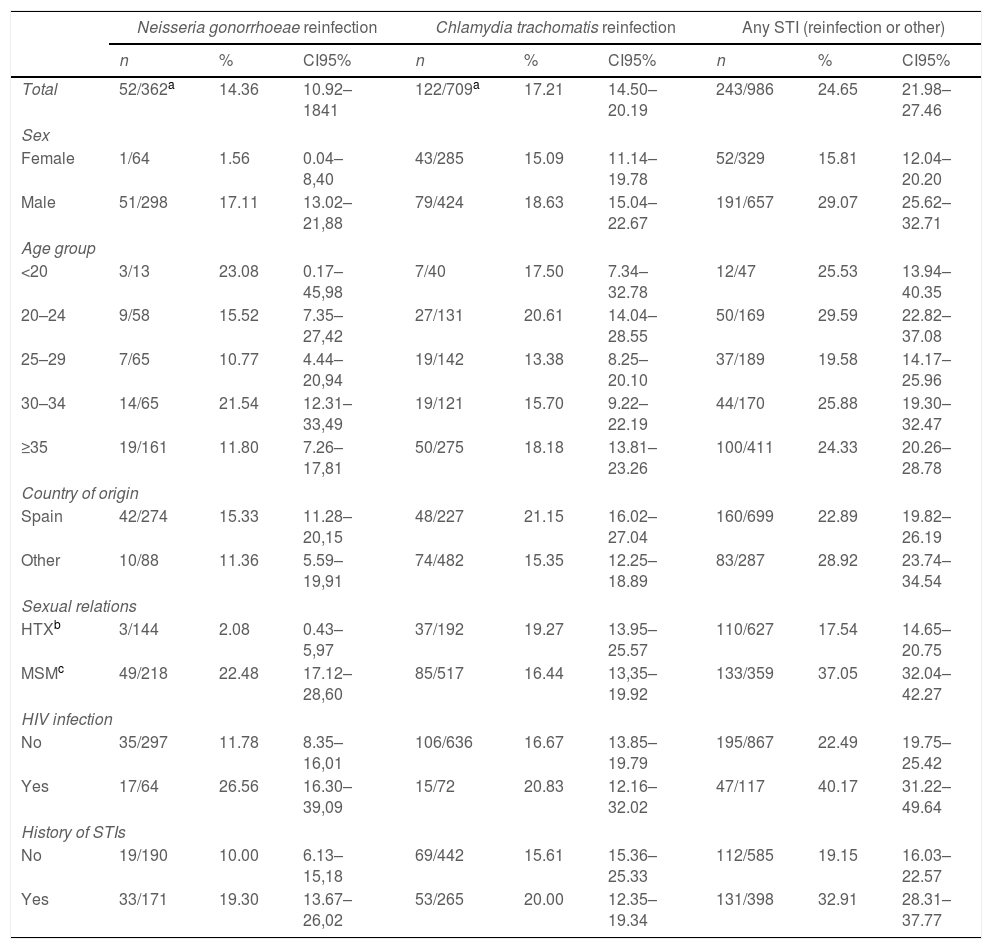
Incidence of reinfections and of STIs (reinfection or other) in rescreeningDuring the six month average follow-up (6263 person-months in total) 243 of the 986 participants were again infected by some STI (accumulated incidence=24.65%; CI95%: 21.98–27.46): 170 patients were reinfected by the same microorganism as the initial one (17.24%; CI95%: 14.93–19.75) and in the remaining 73 (7.4%) the same microorganism was not isolated but a different from the initial one. In 24 of the 170 patients reinfected by the same microorganism, a different one was also isolated. Subsequently, infection with microorganisms other than the initial one was detected in 97 patients (9.8%): syphilis (1.4%), first episode of genital herpes (1.2%), new HIV (0.1%), trichomoniasis (0.4%), escabiosis 0.2%, NG when the initial infection had been CT (2.4%), CT when the initial infection had been a GI (3%) and other non-chlamydial non-gonococcal urethritis (Mycoplasma genitalium [0.5%], Ureaplasma urealyticum [0.6%]).
The six-month specific reinfection incidence by the same microorganism was 14.36% for NG (CI95%: 10.92–18.41) and 17.21% for CT (CI95%: 14.50–20.19) (see Table 2).
Six month incidence of reinfection by Chlamydia tachomatis, Neisseria gonorrhoeae or any STI (reinfection by the same index pathogen or by any other).
| Neisseria gonorrhoeae reinfection | Chlamydia trachomatis reinfection | Any STI (reinfection or other) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | CI95% | n | % | CI95% | n | % | CI95% | |
| Total | 52/362a | 14.36 | 10.92–1841 | 122/709a | 17.21 | 14.50–20.19 | 243/986 | 24.65 | 21.98–27.46 |
| Sex | |||||||||
| Female | 1/64 | 1.56 | 0.04–8,40 | 43/285 | 15.09 | 11.14–19.78 | 52/329 | 15.81 | 12.04–20.20 |
| Male | 51/298 | 17.11 | 13.02–21,88 | 79/424 | 18.63 | 15.04–22.67 | 191/657 | 29.07 | 25.62–32.71 |
| Age group | |||||||||
| <20 | 3/13 | 23.08 | 0.17–45,98 | 7/40 | 17.50 | 7.34–32.78 | 12/47 | 25.53 | 13.94–40.35 |
| 20–24 | 9/58 | 15.52 | 7.35–27,42 | 27/131 | 20.61 | 14.04–28.55 | 50/169 | 29.59 | 22.82–37.08 |
| 25–29 | 7/65 | 10.77 | 4.44–20,94 | 19/142 | 13.38 | 8.25–20.10 | 37/189 | 19.58 | 14.17–25.96 |
| 30–34 | 14/65 | 21.54 | 12.31–33,49 | 19/121 | 15.70 | 9.22–22.19 | 44/170 | 25.88 | 19.30–32.47 |
| ≥35 | 19/161 | 11.80 | 7.26–17,81 | 50/275 | 18.18 | 13.81–23.26 | 100/411 | 24.33 | 20.26–28.78 |
| Country of origin | |||||||||
| Spain | 42/274 | 15.33 | 11.28–20,15 | 48/227 | 21.15 | 16.02–27.04 | 160/699 | 22.89 | 19.82–26.19 |
| Other | 10/88 | 11.36 | 5.59–19,91 | 74/482 | 15.35 | 12.25–18.89 | 83/287 | 28.92 | 23.74–34.54 |
| Sexual relations | |||||||||
| HTXb | 3/144 | 2.08 | 0.43–5,97 | 37/192 | 19.27 | 13.95–25.57 | 110/627 | 17.54 | 14.65–20.75 |
| MSMc | 49/218 | 22.48 | 17.12–28,60 | 85/517 | 16.44 | 13,35–19.92 | 133/359 | 37.05 | 32.04–42.27 |
| HIV infection | |||||||||
| No | 35/297 | 11.78 | 8.35–16,01 | 106/636 | 16.67 | 13.85–19.79 | 195/867 | 22.49 | 19.75–25.42 |
| Yes | 17/64 | 26.56 | 16.30–39,09 | 15/72 | 20.83 | 12.16–32.02 | 47/117 | 40.17 | 31.22–49.64 |
| History of STIs | |||||||||
| No | 19/190 | 10.00 | 6.13–15,18 | 69/442 | 15.61 | 15.36–25.33 | 112/585 | 19.15 | 16.03–22.57 |
| Yes | 33/171 | 19.30 | 13.67–26,02 | 53/265 | 20.00 | 12.35–19.34 | 131/398 | 32.91 | 28.31–37.77 |
In the denominators of these proportions the 85 initial mixed infections have been added to the 277 infections only by NG (total 362) and to the 624 initial infections only by CT (total 709). In the numerator of both proportions have been added 4 initial mixed infections reinfected with both microorganisms.
The probability of being infected by any STI was almost twice as high among those entering the study with a mixed NG-CT infection compared with those entering with a single NG or CT infection (OR: 1.76; IC95%: 1.1–2.8), and the probability of being reinfected by the same microorganism (17.24%; CI95%: 14.93–19.75) also varied according to the initial infection: 13.0% (CI 95%: 9.27–17.54) for those that entered the study with a single infection due to a NG; 16.83% (CI95%: 13.97–20.0) for those initially infected only with CT, and 34.12% (CI95%: 24.18–45.2) for those with mixed infection, a probability approximately three times greater than that of those entering with a single infection (OR: 2.8; IC95%: 1.72–4.52) (see Table 3).
Characteristics associated to the incidence of reinfection or any STI (reinfection by the same index pathogen or by any other) at the rescreening. Logistic regression univariate analysis.
| Reinfection | Any STI (reinfection or other) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | CI95% | OR | CI95% | n | % | CI95% | OR | CI95% | |
| Index infection | ||||||||||
| Neisseria gonorrhoeae only | 36/277 | 13.0 | 09.27–17.54 | Ref. | 69/277 | 24.91 | 19,93–30,44 | Ref. | ||
| Chlamydia trachomatis only | 105/624 | 16.83 | 13.97–20.00 | 1.354 | 0.900–2.037 | 144/624 | 23.08 | 19,82–26,59 | 0.904 | 0.650–1.258 |
| Both (mixed infection) | 29/85 | 34.12 | 24.18–45.20 | 3.467 | 1.963–6.124 | 30/85 | 35.29 | 25,23–46,41 | 1.645 | 0.977–2.771 |
| Sex | ||||||||||
| Female | 44/329 | 13.7 | 09.89–17.54 | Ref. | 52/329 | 15.81 | 12,04–20.20 | Ref. | ||
| Male | 126/657 | 19.18 | 16.24–22.40 | 1.537 | 1.059–2.229 | 191/657 | 29.07 | 25,62–32,71 | 2.183 | 1.553–3.070 |
| Country of origin | ||||||||||
| Spain | 113/699 | 16.17 | 13.51–19.11 | Ref. | 160/699 | 22.89 | 19,82–26,19 | Ref. | ||
| Other | 57/287 | 19.86 | 15.40–24.95 | 1.285 | 0.903–1.829 | 83/287 | 28.92 | 23,74–34,54 | 1.371 | 1.005–1.869 |
| Age | ||||||||||
| <20 | 10/47 | 21.28 | 10.70–35.66 | Ref. | 12/47 | 25.53 | 13.94–40.35 | Ref. | ||
| 20–24 | 35/169 | 20.71 | 14.87–27.61 | 0.966 | 0.438–2.132 | 50/169 | 29.59 | 22.82–37.08 | 1.226 | 0.588–2.554 |
| 25–29 | 25/189 | 13.23 | 08.75–18.90 | 0.564 | 0.250–1.275 | 37/189 | 19.58 | 14.17–25.96 | 0.710 | 0.336–1.500 |
| 30–34 | 32/170 | 18.82 | 13.25–25.52 | 0.858 | 0.386–1.904 | 44/170 | 25.88 | 19.30–32.47 | 1.019 | 0.486–2.135 |
| ≥35 | 68/411 | 16.55 | 13.08–20.50 | 0.733 | 0.348–1.546 | 100/411 | 24.33 | 20.26–28.78 | 0.938 | 0.469–1.876 |
| Sexual relations | ||||||||||
| HTXa | 88/627 | 14.04 | 11.41–17.00 | Ref. | 110/627 | 17.54 | 14.65–20.75 | Ref. | ||
| MSMb | 82/359 | 22.84 | 18.60–27.54 | 1.813 | 1.298–2.532 | 133/359 | 37.05 | 32.04–42.27 | 2.766 | 2.055–3.723 |
| Abstention from sex for a week from the start of treatment | ||||||||||
| Yes | 137/858 | 15.97 | 13.58–18.59 | Ref. | 198/850 | 23.08 | 20.30–26.04 | Ref. | ||
| No | 13/58 | 22.41 | 12.51–35.27 | 1.520 | 0.798–2.893 | 16/58 | 27.59 | 16.66–40.90 | 1.269 | 0.698–2.307 |
| Unknown | 70 | 70 | ||||||||
| Compliance whit partner notification | ||||||||||
| Yes | 80/505 | 15.84 | 12.77–19.32 | Ref. | 105/505 | 20.79 | 17.33–24.60 | Ref. | ||
| No | 74/427 | 17.33 | 13.86–21.26 | 1.114 | 0.788–1.574 | 114/427 | 26.70 | 22.56–31.16 | 1.387 | 1.024–1.880 |
| Unknown | 54 | 54 | ||||||||
| Partners since treatment | ||||||||||
| 0–1 | 68/576 | 11.81 | 09.29–14.73 | Ref. | 83/576 | 14.41 | 11.64–17.55 | Ref. | ||
| 2–5 | 61/271 | 22.51 | 17.68–27.95 | 2.170 | 1.482–3.177 | 93/271 | 34.32 | 28.68–40.30 | 3.103 | 2.204–4.369 |
| >5 | 39/133 | 29.32 | 21.75–37.84 | 3.100 | 1.974–4.866 | 64/133 | 48.12 | 39.38–56.95 | 5.509 | 3.649–8.318 |
| Unknown | 8 | 8 | ||||||||
| Steady partner at the rescreening visit | ||||||||||
| Yes | 88/560 | 15.71 | 12.80–19.00 | Ref. | 120/560 | 21.43 | 18.10–25.06 | Ref. | ||
| No | 82/426 | 19.25 | 15.61–23.32 | 1.279 | 0.918–1.78 | 123/426 | 28.87 | 24.61–33.43 | 1.488 | 1.113–1.991 |
| New steady partner since treatment | ||||||||||
| No | 140/858 | 16.32 | 13.91–18.96 | Ref. | 202/858 | 23.54 | 20.74–26.53 | Ref. | ||
| Si | 29/125 | 23.20 | 16.12–31.59 | 1.549 | 0.985–2.437 | 40/125 | 32.00 | 23.94–40.93 | 1.529 | 1.017–2.298 |
| Unknown | 3 | 3 | ||||||||
| Condom use since treatment | ||||||||||
| Always | 68/475 | 14.32 | 11.29–17.79 | Ref. | 105/475 | 22.32 | 18.65–26.33 | |||
| Sometimes/never | 94/483 | 19.46 | 16.02–23.28 | 1.446 | 1.028–2.035 | 127/483 | 26.29 | 22.42–30.46 | 1.242 | 0.924–1.670 |
| Unknown | 28 | 28 | ||||||||
| History of STIs (other than HIV) | ||||||||||
| No | 87/586 | 14.85 | 12.07–17.99 | Ref. | 112/586 | 19.11 | 16.01–22.53 | Ref. | ||
| Yes | 83/399 | 20.80 | 16.92–25.12 | 1.507 | 1.081–2.100 | 131/399 | 32.83 | 28.24–37.68 | 2.069 | 1.543–2.774 |
| Unknown | 1 | 1 | ||||||||
| HIV infection | ||||||||||
| No | 139/869 | 16.00 | 13.62–18.60 | Ref. | 196/869 | 22.55 | 19.82–25.48 | Ref. | ||
| Yes | 31/117 | 26.50 | 18.77–35.45 | 1.893 | 1.208–2.966 | 47/117 | 40.17 | 31.22–49.64 | 2.305 | 1.542–3.448 |
| Drugs use prior to sex since treatment | ||||||||||
| No | 158/937 | 16.86 | 14.52–.1942 | Ref. | 221/937 | 23.59 | 20.90–26.44 | Ref. | ||
| Yes | 9/39 | 23.08 | 11.13–39.33 | 1.480 | 0.689–3.178 | 18/39 | 46.15 | 30.09–62.82 | 2.778 | 1.454–5.30 |
| Unknown | 10 | 10 | ||||||||
| Alcohol use prior to sex since treatment | ||||||||||
| No | 161/949 | 16.97 | 14.63–19.51 | Ref. | 220/949 | 23.18 | 20.53–26.00 | Ref. | ||
| Yes | 6/25 | 24.00 | 09.36–45.13 | 1.547 | 0.608–3.933 | 17/25 | 68.00 | 46.50–85.05 | 7.037 | 2.997–16.524 |
| Unknown | 12 | 12 | ||||||||
| Drugs and/or alcohol use prior to sex since treatment | ||||||||||
| No | 153/920 | 16.63 | 14.28–19.20 | Ref. | 207/920 | 22.50 | 19.84–25.34 | Ref. | ||
| Yes | 14/54 | 25.93 | 14.96–39.65 | 1.755 | 0.932–3.304 | 30/54 | 55.56 | 41.40–69.08 | 4.306 | 2.463–7.527 |
| Unknown | 12 | 12 | ||||||||
| Sex worker | ||||||||||
| No | 166/969 | 17.13 | 14.81–19.65 | Ref. | 239/969 | 24.66 | 21.98–27.50 | Ref. | ||
| Yes | 4/17 | 23.53 | 06.81–49.90 | 1.489 | 0.480–4.624 | 4/17 | 23.53 | 06.81–49.90 | 0.941 | 0.304–2.912 |
The great majority, 74% of those who had a reinfection by the same microorganism and 72.8% of those who presented any STI (reinfection or other) were asymptomatic when they were rescreened.
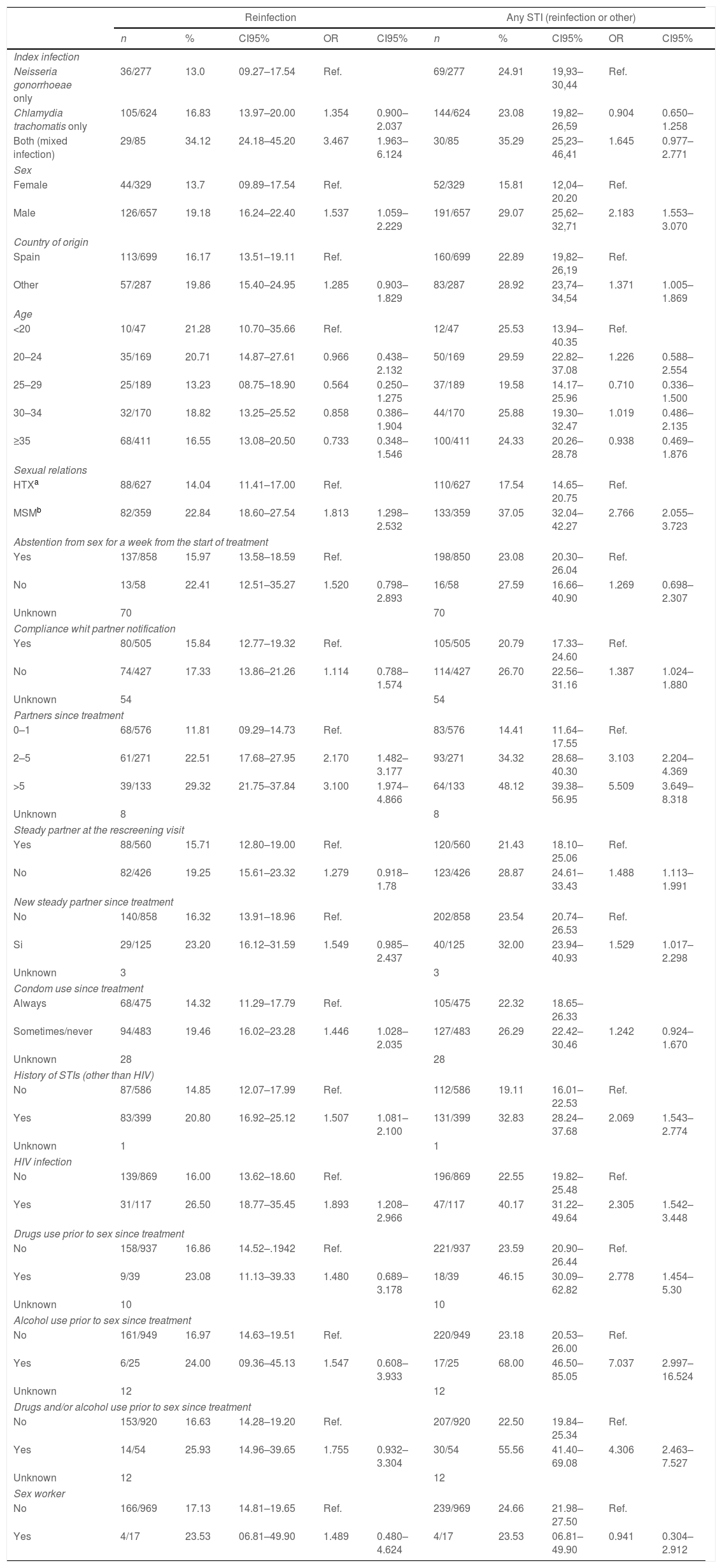
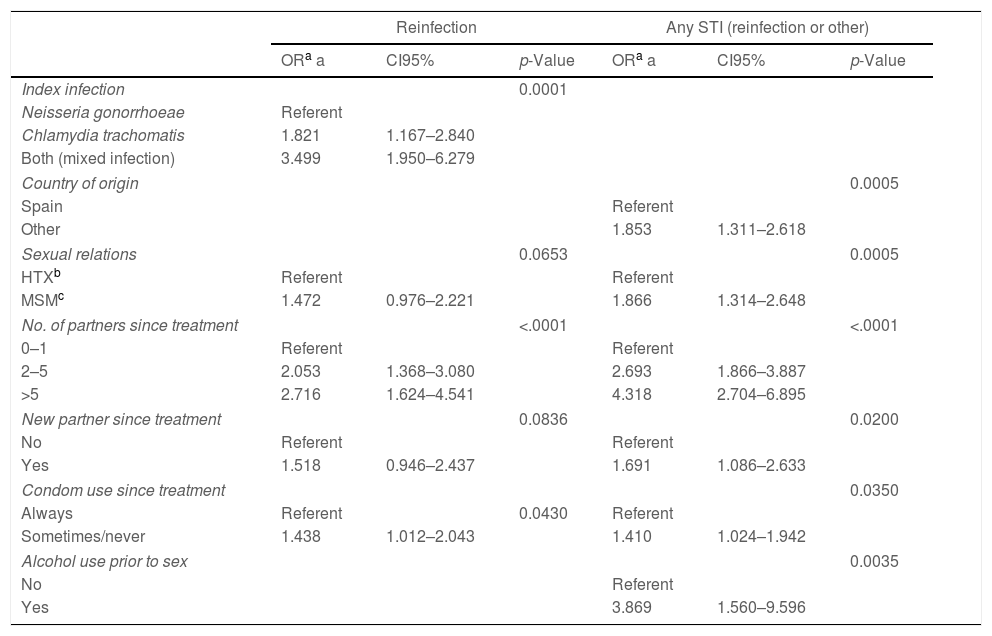
Tables 3 and 4 present, respectively, the raw ORs and those adjusted after simultaneously controlling for the study variables, resulting from the statistical models that examine the association of the different patient characteristics with reinfection and with having an STI (reinfection or other) in the rescreening. With respect to reinfection by the same microorganism: the number of sexual partners since the treatment (OR=2.05; CI95%: 1.3–3.0 for 2–5 sexual partners with respect to 0–1 partners and OR=2.7; CI95%: 1.6–4.5 for those who had more than 5 partners), not always using a condom in vaginal/anal sex (OR=1.4; CI95%: 1.01–2.04) and the type of initial infection, were associated independently with the probability of being reinfected by the same microorganism. Those who had a CT infection had nearly twice as much probability of reinfection as those who had a GI (OR=1.8; CI95%: 1.16–2.8) and the probability was even greater in those who had a mixed infection (OR=3.5; CI95%: 1.9–6.2) (see Table 4).
Risk factors for reinfection or any STI (reinfection by the same index pathogen or by any other) at the rescreening. Logistic regression multivariate analysis.
| Reinfection | Any STI (reinfection or other) | |||||
|---|---|---|---|---|---|---|
| ORa a | CI95% | p-Value | ORa a | CI95% | p-Value | |
| Index infection | 0.0001 | |||||
| Neisseria gonorrhoeae | Referent | |||||
| Chlamydia trachomatis | 1.821 | 1.167–2.840 | ||||
| Both (mixed infection) | 3.499 | 1.950–6.279 | ||||
| Country of origin | 0.0005 | |||||
| Spain | Referent | |||||
| Other | 1.853 | 1.311–2.618 | ||||
| Sexual relations | 0.0653 | 0.0005 | ||||
| HTXb | Referent | Referent | ||||
| MSMc | 1.472 | 0.976–2.221 | 1.866 | 1.314–2.648 | ||
| No. of partners since treatment | <.0001 | <.0001 | ||||
| 0–1 | Referent | Referent | ||||
| 2–5 | 2.053 | 1.368–3.080 | 2.693 | 1.866–3.887 | ||
| >5 | 2.716 | 1.624–4.541 | 4.318 | 2.704–6.895 | ||
| New partner since treatment | 0.0836 | 0.0200 | ||||
| No | Referent | Referent | ||||
| Yes | 1.518 | 0.946–2.437 | 1.691 | 1.086–2.633 | ||
| Condom use since treatment | 0.0350 | |||||
| Always | Referent | 0.0430 | Referent | |||
| Sometimes/never | 1.438 | 1.012–2.043 | 1.410 | 1.024–1.942 | ||
| Alcohol use prior to sex | 0.0035 | |||||
| No | Referent | |||||
| Yes | 3.869 | 1.560–9.596 | ||||
aOR a: adjusted odds ratio.
The risk factors for incidence rates of STI (reinfection by the same microorganism or by another) are the following: being an immigrant (OR=1.8; CI95%: 1.3–2.6), MSM (OR=1.8; CI95%: 1.3–2.6), number of sexual partners since the treatment (OR=4.3; CI95%: 2.7–6.8 for those who had more than 5 partners with respect to those who had 0–1 partners), having a new partner (OR=1.7; CI95%: 1.08–2.6), not always using a condom in genital sex (OR=1.4; CI95%: 1.02–1.9) and consumption of alcohol in relation to sex (OR=3.8; CI95%: 1.5–9.5) (see Table 4).
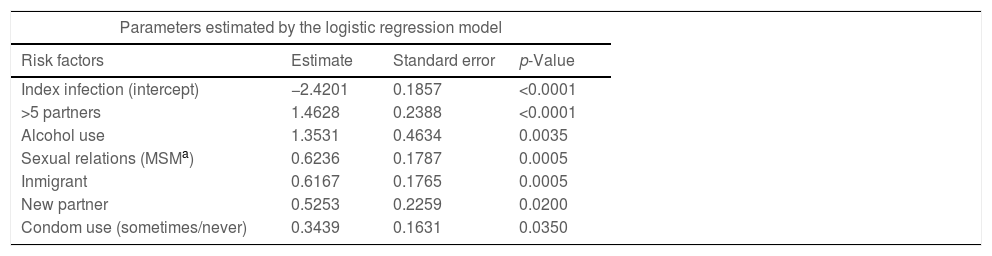
In accordance with the coefficients of the multivariate statistical model the probability of reinfection or another STI in six months can be predicted, as shown in Table 5. For example, this probability is 14% in the case of being an immigrant or MSM. If we add other risk factors, such as having had more than five sexual partners in recent months to any of these characteristics, it goes up to 41%. If we bring together four factors: MSM, immigrant, more than five partners and alcohol, the probability surpasses 80%. This risk increases linearly as the seven indicated risk factors are accumulated, reaching 92.5% in those that combine all of them.
Probability of any STI (reinfection or other) at six months from the index infection based on identified risk factors.
| Parameters estimated by the logistic regression model | |||
|---|---|---|---|
| Risk factors | Estimate | Standard error | p-Value |
| Index infection (intercept) | −2.4201 | 0.1857 | <0.0001 |
| >5 partners | 1.4628 | 0.2388 | <0.0001 |
| Alcohol use | 1.3531 | 0.4634 | 0.0035 |
| Sexual relations (MSMa) | 0.6236 | 0.1787 | 0.0005 |
| Inmigrant | 0.6167 | 0.1765 | 0.0005 |
| New partner | 0.5253 | 0.2259 | 0.0200 |
| Condom use (sometimes/never) | 0.3439 | 0.1631 | 0.0350 |
| Joint probability according to risk factors combination | ||
|---|---|---|
| Probability | CI95% | |
| NGb and/or CTc infection without other risk factors | 08.16 | 05.82–11.34 |
| + >5 partners | 27.74 | 18.94–38.68 |
| + >5 partners+alcohol use | 59.76 | 34.94–80.42 |
| + >5 partners+alcohol use+MSM | 73.48 | 51.71–87.76 |
| + >5 partners+alcohol use+MSM+inmigrant | 83.70 | 66.01–93.14 |
| + >5 partners+alcohol use+MSM+inmigrant+new partner | 89.67 | 74.85–96.20 |
| + >5 partners+alcohol use+MSM+inmigrant+new partner+condom use (sometimes/never) | 92.45 | 80.87–97.26 |
Our results show that the risk of reinfection among those who have had a GI or a CT infection is 13% and 16.8% respectively, reaching 34% among those who initially had a mixed infection. Patients with CT were almost twice as likely to be reinfected than those with GI, and those with a mixed infection 3.5 times more. If we consider not only reinfection by the same microorganism, but also the fact of repeated STI, either the same STI as at the start of the study or another, the risk of repeated infection is 25%. Among those who initially had a mixed infection, this risk increases to 35%. These figures suggest a relative failure in the management of the STIs. Despite receiving appropriate treatment, information on their infection, the need to abstain from sex for a week from the start of treatment and the reasons for studying their sexual contacts, advice on safe sex, and having accepted re-evaluation, one in four patients re-contracted a STI over an average of six months.
With respect to other studies conducted in Spain, the estimated accumulated incidence of CT reinfection is slightly lower than that obtained by this same team in a previous retrospective study: 17.2 vs. 18.3.13 If we limit ourselves to women under 25, our estimate (19.10%; CI95%: 11.54–28.81) nearly doubles that of López-Corbeto et al. in a sample of 29 women in Cataluña.14
The review by Hosenfeld et al.3 of 16 prospective studies in women, conducted before 2008 in different countries, reported a CT reinfection incidence similar to that of our study (15%): 14.7% at six months from the initial infection. Subsequent prospective studies have reported reinfection proportion in women, generally under 30 years of age, between 8.6% and 25.5%.16–20 In the case of men, the observed incidence in our study (18.63%) is greater than that reported in the review by Fung et al.4 of eight prospective studies between 1995 and 2006, with a median reinfection of 10.9% and that of other subsequent studies that obtained figures between 9.2% and 13%.16,21
Prospective studies of gonococcal reinfections are more scarce. In the review by Hosenfeld et al.3 gonococcal reinfection incidence in women varied from 3.6% to 40% (median 23.6%) and in men, the review by Fung et al.4 reported rates between 0 and 30.8% (median 7%). Subsequent retrospective studies report rates between 6.5–15.6% in women and 13.7–23% in men.22–24 Incidence in women is very low in our work (1.56%) compared to these studies, while that of men (17.1%) is among the highest of those reported.
In the current situation, with STI clinics overworked in a context of limited resources, our model can be useful for establishing priorities, selecting high-risk patients (MSM, immigrants, more than five sexual partners in recent months, alcohol use, new partner, condom use occasionally/never) for specific prevention and control interventions such as STI rescreening. In these patients it is necessary to carry out comprehensive STI screening, collecting samples from all the locations susceptible to infection and conducting serological tests, since it deals not only with detecting an NG or CT reinfection, but also other possible infections (syphilis, HIV, trichomoniasis, etc.).
We observed an increase in the use of condoms between the treatment and the rescreening (from 25.6% to 49.6%). This is in line with the findings of other studies that have shown an increase in the use of condoms after the STI diagnosis, but it seems to be a temporary effect.25 Although the advice on safe sex must be part of any sexual health consultation, we lack evidence that clearly shows its effectiveness in reducing the STI incidence rate1,26 and more research is needed to know how to help people to change their sexual behaviour and to practice safer sex. At present, treatment is the most effective preventive strategy for STI control. When we make an early diagnosis and treatment, we are making primary prevention of the transmission at the population level and secondary prevention of possible individual complications.27 Rescreening allows early diagnosis and treatment of infections, which are mostly asymptomatic, reducing the risk of transmission and complications. We lost 17% of the participants in our study. It is important to establish mechanisms not to lose patients with a high risk of infection by active reminders of their appointments (telephone calls, mobile phone messages, or others).
In 46% of the cases no contact could be studied. Currently, the most common way, in our setting, to inform sexual contacts of persons with STIs of their potential exposure to infection and to offer them evaluation and treatment is through “patient referral” which can be limited for multiple reasons. This means that many people will continue to spread the infection without knowing it. It is necessary to evaluate the implementation of other methods of contact notification through the use of new technologies and to assess the regulation of patient-delivered partner treatment.28
This study's principal limitation is that it is based on patients treated in STI clinics, and its extrapolation to the general population must be done with caution. Even so, our clinics are those of reference for STIs in the public health system and provide clinical care for up to 90% for gonorrhoea cases and more than 82% for those with CT infections reported to the Health Department of Biscay. Therefore, we believe that, lacking population-based studies, our results can be generalized reasonably to our target population. Regarding the diagnosis in the re-screening of infections other than the initial one, M. genitalium study was only conducted in non-chlamydial non-gonococcal urethritis in men, which means that the proportion of isolates of this microorganism is underestimated. In any case, routine screening of asymptomatic M. genitalium infection among women and men or extragenital testing for M. genitalium is not recommended.29 Therefore, we consider that this under-diagnosis does not significantly affect our estimate of the incidence of any STI in the re-screening. Finally, 17% of the participants did not complete the study, so we do not know whether they were reinfected or not.
The study's principal strength lies in being the first prospective study conducted in Spain, with nearly 1000 patients, which estimates the CT and NG reinfection incidence. The majority of studies focus on specific populations: young women, in the case of CT reinfections or NG in men. This paper includes an extensive sample of both genders between 14 and 72 years of age. In addition we have estimated the incidence of recurrent STI, by the same microorganism or by a different one from that which motivated patients’ entry into the study. This delivers a stronger outcome for identifying the socio-demographic and behavioural characteristics of those in whom the repetition of detection tests will be most efficient.
From the public health point of view, our results must not leave us satisfied. We must share this information with patients, discuss how we could tackle the alarmingly high recurrence of these infections, and actively involve them in designing strategies to reduce their incidence. Otherwise, given the growing rate and our limited effectiveness, the global epidemic of STI will continue creating more and greater problems. A hundred years ago Ernest Codman sentenced: “Every hospital should follow every patient it treats, long enough to determine whether or not the treatment has been successful, and then to inquire, ‘if not, why not’ with a view to preventing similar failure in future”.30 Each reinfection is a failure.
Source of fundingThis work has been funded by the Department of Health of the Basque Government (file 2015111136).
Conflict of interestNone of the authors has any conflict of interest in relation to the information presented in this manuscript.
We thank Gonzalo Grandes Odriozola, Head of the Primary Care Research Unit of Biscay, Basque Healthcare Service, Biocruces-Bizkaia Research Institute, for reviewing the manuscript.