Nosocomial meningitis is a devastating condition complicating invasive procedures of the cranium.1 Carbapenem-resistant Enterobacterales (CRE) meningitis is an emerging healthcare problem2 not only due to limited antibiotic options1 but also due to the lack of penetration of antimicrobials in cerebrospinal fluid (CSF).1 Ceftazidime-avibactam (CAZ/AVI) is a new antimicrobial developed for the treatment of certain CRE. Experience with CAZ/AVI in the treatment of central nervous system (CNS) infections due to CRE is scarce.3–6
Here we present a case of an otherwise healthy 62-year-old man with a right-sided cerebellar hematoma and communicated hydrocephalus, managed with an extra-ventricular drainage (EVD). On the 18th day of admission, he developed carbapenem-resistant Klebsiella pneumoniae (CRKP) bacteremia. Meningitis was ruled-out at that time as a result of normal CSF findings. Antibiotic susceptibility of the CRKP was as follows: MICs (mg/L) of meropenem, amikacin, trimethoprim/sulfamethoxazole, ciprofloxacin, colistin, and fosfomycin were 32, >16, >4/76, >2, 8, and 4, respectively. Treatment was initiated with meropenem 1grTID, colistimethate sodium 150mgBID and ertapenem 1gr (creatinine clearance: 40ml/min). Since his clinical condition deteriorated, fosfomycin 4mgQD was added to the previous antibiotic regimen. The clinical course of the patient was further complicated with bacterial meningitis and CSF cultures yielded CRKP on the 24th day of his admission. The dose of meropenem was increased to 2gTID, the drainage catheter was changed and colistimethate sodium 10mgQD was administered intrathecally (Supplemental Fig. 1). However, the patient remained febrile and his mental status worsened. Despite double carbapenem plus colistin plus intrathecal interventions, CSF cultures kept growing CRKP until the 36th day of admission. Discontinued intrathecal colistin and initiated treatment with intrathecal amikacin 15mgTID on the 36th day of the admission while a ventriculoperitoneal shunt was placed owing to a drainage asymmetry. Under this treatment CSF protein level increased to a peak of 229mg/dl while CSF glucose decreased to its nadir of 18mg/dl. An abscess formation was visualized in a contrast-enhanced magnetic resonance study (Fig. 1). Multiplex RT-PCR confirmed that the CRKP carried an OXA-48-carbapenemase, being negative for NDM, KPC, VIM and IMP.7 Antimicrobial susceptibility of CAZ/AVI (30/20mcg) by disk diffusion demonstrated a 20mm inhibition zone. Bacterial CSF cultures remained positive for the CRKP, and treatment with CAZ/AVI plus trimethoprim-sulfamethoxazole plus intrathecal amikacin were initiated on the 45th day of his admission after obtaining official approval from Ministry of Health (IRB approval: REIYS 19/3/201-2019-03-188576). The treatment was then simplified to CAZ/AVI monotherapy (2.5gTID) on the 60th day of admission. CSF analysis showed a gradual decrease in CSF protein from 229mg/dl to 121mg/dl and to 64mg/dl, while CSF glucose increased from 18mg/dl to 72mg/dl. The neurologic status of the patient improved and he was transferred from critical care to the ward on the 65th day of his admission. Repeated CSF cultures obtained from the EVD remained sterile, EVD was withdrawn and CAZ/AVI treatment was discontinued on the 75th day of his admission. He was able to understand complicated orders, speak, and mobilize by one sided assistance.
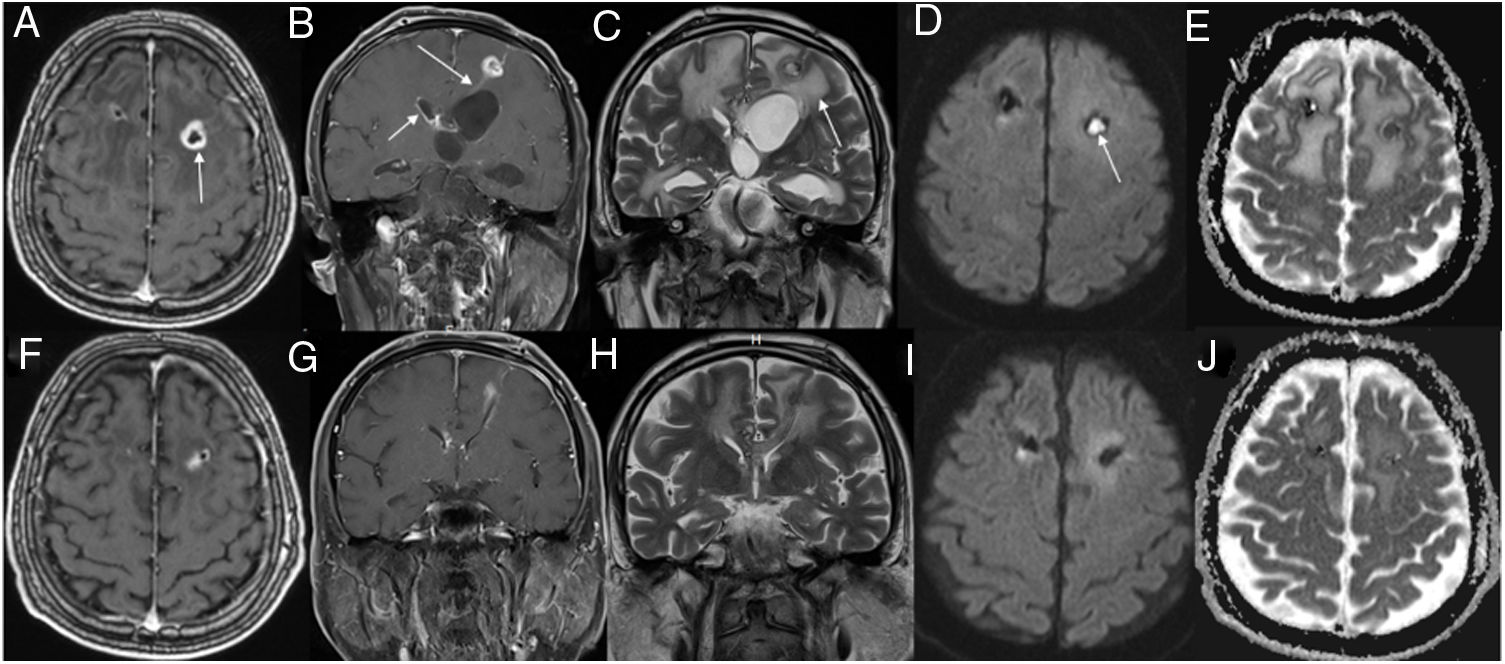
Top rows show initial magnetic resonance imaging (MRI) and bottom rows show follow-up MRI. Axial (A) and coronal (B) contrast-enhanced T1-weighted (W) images show a ring-enhancing lesion (arrow) in the left frontal region, with enhancement along the ventriculostomy tract (B, long arrow). Also, note enhancement of the ependyma (B, short arrow) and surrounding edema (C, arrow). Trace diffusion-weighted image (DWI) shows central brightness in the lesion (D, arrow) and a low ADC indicating restricted diffusion (E). These imaging findings are consistent with brain abscess. Three weeks after antibiotherapy, contrast-enhanced T1W images (F, G) show that the lesion is diminished, with predominantly low signal intensity on a DWI (I) and a high ADC on ADC map (J); these findings suggest clear fluid in the abscess cavity.
Nosocomial meningitis can complicate 8% of cases treated with EVD.1 Carbapenem resistance in gram negative bacteria further impair antimicrobial penetration through the outer bacterial wall which in turn results in decreased antimicrobial effectiveness.8 That is why, there are only few antibiotic options left for the treatment of CRKP meningitis with limited success.1,9 In a recent study 76.8% of CRKP infections were found to be resistant to colistin and the survival rate with combination therapies including colistin was significantly shorter than without colistin.10 Addition of intrathecal colistin also decreased the fatality rate when compared to monotherapy with intravenous colistin (13% vs 72%, p=0.001).11 Some studies state that intrathecal amikacin can be used instead of intrathecal colistin.12 Another option is the double carbapenem approach which reduced CRKP bacterial load and mortality in experimental studies.13 Fosfomycin, is another option for the treatment of CRKP meningitis, albeit only one case has been reported until now.14 Bacterial virulence factors,1 bacterial resistance mechanisms and CSF penetration of antimicrobials1 are the main factors influencing this success diversity. The isolation of CRKP-OXA-48, carrying a plasmid encoding this class D carbapenemase, has dramatically increased over the last fifteen years.15 It is known that CAZ/AVI might be used in CRE infections, since avibactam inhibits class A, C and D beta-lactamases.3 In addition, avibactam CSF penetration was found to be 38% in animal models and also might be responsible for this effect.3 It is known that almost 90% of the protein non-bound free fraction of avibactam is responsible for its pharmacodynamic effect.16 Limited number of case reports of CRE meningitis/ventriculitis successfully treated with CAZ/AVI have been recently published in literature3–5 (Supplemental Table 1). In all cases the shared feature was an implantation of a foreign material into the CNS. As a result, CRE meningitis is an emergent phenomenon especially after neurosurgical procedures in critical care circumstances. Herein we described a CRKP meningitis after an EVD implantation which was successfully treated with intravenous CAZ/AVI.
Patient consentThe patient has on his own consented to the submission of the case report for submission to the journal.
ICMJE statementMYP and ACI were responsible for the organization and coordination of the study. MYP and ACI as the chief investigator and responsible for the data analysis. All authors contributed to the writing of the final manuscript.
FundingNone.
Conflict of interestNo author has stated any conflict of interest.