The emergence of multidrug-resistant (MDR) Mycobacterium tuberculosis strains has become a worldwide health care problem, making treatment of tuberculosis difficult. The aim of this study was to determine phenotypic resistance and gene mutations associated with MDR of clinical isolates of Mycobacterium tuberculosis from Guadalajara, Mexico.
MethodsOne hundred and five isolates were subjected to drug susceptibility testing to first line drugs using the proportion and Mycobacteria Growth Indicator Tube (MGIT) methods. Genes associated with isoniazid (inhA, katG, ahpC) and rifampicin (rpoB) resistance were analyzed by either pyrosequencing or PCR-RFLP.
ResultsResistance to any drug was detected in 48.6% of isolates, of which 40% were isoniazid-resistant, 20% were rifampicin-resistant and 19% were MDR. Drug-resistant isolates had the following frequency of mutations in rpoB (48%), katG (14%), inhA (26%), ahpC (26%). Susceptible isolates also had a mutation in ahpC (29%).
ConclusionsThis is the first analysis of mutations associated with MDR of M. tuberculosis in Guadalajara. Commonly reported mutations worldwide were found in rpoB, katG and inhA genes. Substitution C to T in position -15 of the ahpC gene may possibly be a polymorphism.
La emergencia de cepas de Mycobacterium tuberculosis multifarmacorresistentes (MFR) se ha convertido en un problema de salud mundial, dificultando el tratamiento de la tuberculosis. El objetivo de este estudio fue determinar la resistencia fenotípica y las mutaciones en genes asociados a MFR en aislamientos clínicos de M. tuberculosis de Guadalajara, México.
MétodosSe determinó la susceptibilidad a fármacos de primera línea de 105 aislamientos, usando los métodos de proporciones y MGIT. Los genes asociados a resistencia a isoniazida (inhA, katG, ahpC) y a rifampicina (rpoB), se analizaron por pirosecuenciación o por PCR-RFLP.
ResultadosSe detectó la resistencia a cualquier fármaco en 48.6% de los aislamientos, 40% fueron resistentes a isoniazida, 20% fueron resistentes a rifampicina y 19% fueron MFR. Los aislamientos farmacorresistentes presentaron la siguiente frecuencia de mutaciones en rpoB (48%), katG (14%), inhA (26%), ahpC (26%). Además, los aislamientos susceptibles también mostraron una mutación en ahpC (29%).
ConclusionesEste es el primer análisis de mutaciones asociadas con MFR de M. tuberculosis en Guadalajara. Se detectaron mutaciones comúnmente reportadas a nivel mundial en los genes rpoB, katG e inhA. La sustitución de C por T en la posición -15 del gen ahpC posiblemente puede ser un polimorfismo.
Tuberculosis (TB) is an important death-causing disease, considered to be the second leading cause of death from an infectious disease worldwide. Despite the availability of highly efficacious treatment for decades, TB remains a major global health problem. The World Health Organization (WHO) estimates that about one-third of the global population is infected with Mycobacterium tuberculosis.1 In Mexico, more than two thousand people die of this disease every year and the TB rate per 100,000 population was 16.8 in 2010. In Jalisco, the TB rate was 13 per 100,000 population.2 These data render tuberculosis an important public health problem in this state that deserves special attention.
One of the most alarming trends concerning TB is the emergence of multidrug-resistant strains of M. tuberculosis (MDR-TB), defined as resistant to at least isoniazid (INH) and rifampicin (RIF), which has been steadily increasing over the years, making treatment of TB difficult. Thus, it is important to identify resistant strains as soon as possible to allow adjustments in treatment and minimize transmission. Drug resistance (DR) in M. tuberculosis is caused by mutations in conserved regions of the genome. RIF-resistance is mainly caused by mutations in the β subunit of the RNA polymerase, which is encoded by the rpoB gene. More than 95% of resistant strains harbor mutations within the rifampicin resistance determining region (RRDR), an 81-bp hot-spot (codons 507–533) of rpoB, the most common of which occur at codons 526 and 531. Mutations in the catalase peroxidase gene (katG) and in a gene encoding the enoyl acyl carrier protein reductase (inhA) have been found to account for 40–60% and 20–34% of INH-resistant M. tuberculosis strains, respectively. Similarly, mutations in the intergenic region oxyR-ahpC can reduce the level of expression of inhA and have been associated with INH-resistance (10–15%).3,4
DR tuberculosis is a serious public health issue, especially in developing countries. To solve this problem it is necessary to carry out surveillance of resistance patterns and the mutations associated to this resistance. Some studies in Mexico have analyzed the frequency of gene mutations associated with drug resistance of clinical isolates of M. tuberculosis.5–15 Guadalajara, Jalisco is one region that has been not studied. The aim of this study is to determine phenotypic resistance to first-line drugs and gene mutations associated with INH and RIF resistance of clinical isolates of M. tuberculosis in Guadalajara, Mexico.
Materials and methodsClinical specimens and isolatesThe Hospital Civil de Guadalajara, Fray Antonio Alcalde, admits >240 patients suspected of having tuberculosis per year (2005–2011). Beginning in September 2010 to November 2011, three hundred and fifty one specimens were collected. Samples were decontaminated by modified Petroff's method and cultured on Löwestein-Jensen slants at 37°C. Smears were made and stained by the Ziehl Neelsen staining method and examined for the presence of acid-fast bacilli (AFB). The resulting isolates were identified by traditional biochemical tests, niacin production and nitrate reduction. The local ethics committee approved this study (MB11-005) and informed consent was obtained from patients.
DNA isolationA loopful of bacterial colony suspended in 1mL of sterile water was first inactivated at 80°C for 1h. The suspensions were then centrifuged at 8000rpm for 5min. Supernatants were discarded and pellets resuspended in 200μL of 100mM Tris–HCl and incubated with lysozyme (1mg/mL) at 37°C overnight followed by incubation with 1% SDS and proteinase K (10mg/L) at 55°C for 2h. DNA was then extracted using a phenol extraction, ethanol precipitation method.16
Molecular identification of M. tuberculosis strainsSpecies identification was performed using multiplex PCR amplification of cfp32, RD9 and RD12.17,18 Primer pairs for cfp32 (5′-ATGCCCAAGAGAAGCGAATACAGGCAA-3′ and 5′-CTATTGCTGCGGTGCGGGCTTCAA-3′), RD9 (5′-TCGCCGCTGCCAGATGAGTC-3′ and 5′-TTTGGGAGCCGCCGGTGGTGATGA-3′) and RD12 (5′-GTCGGCGATAGACCATGAGTCCGTCTCCAT-3′ and 5′-GCGAAAAGTGGGCGGATGCCAG-3′) were used as a primer mixture for three simultaneous PCRs in one tube. The reaction mixture contained 1X PCR buffer, 2mM MgCl2, 0.2mM of each dNTP, 2.5μL of dimethyl sulfoxide, 750nM each of cfp32 primers (Rv0577F and Rv0577R), 250nM each of RD primers (Rv2073cF, Rv2073cR, Rv3120F and Rv3120R), 1U of AmpliTaq polymerase (Bioline USA Inc., Randolph, MA, USA) and 200ng of DNA. PCR was initiated by denaturation for 1min at 96°C, followed by 35 cycles of 10s at 96°C, 20s at 60°C and 1min at 72°C with final extension for 5min at 72°C. With this PCR, an isolate possessing all three regions can be identified as M. tuberculosis.
Phenotypic drug susceptibility testingDrug susceptibility testing (DST) of all M. tuberculosis isolates was performed using two methods. The indirect proportion method on Löwestein-Jensen slants was performed as described previously with the following critical concentrations: STR 4.0μg/mL, INH 0.2μg/mL, RIF 40μg/mL, EMB 2.0μg/mL.19 Drug resistance was expressed as the proportion of colonies that grow on drug containing medium to drug-free medium and the critical proportion for resistance was 1% for all drugs. The manual MGIT method was performed using the following critical concentrations: STR 0.8μg/mL, INH 0.1μg/mL, RIF 1.0μg/mL, EMB 3.5μg/mL, according to the protocol provided by the manufacturers (Becton Dickinson, Sparks, MD).20 Fluorescence indicating microbial growth was detected with a 365-nm UV light from a transiluminator. When discordant results were found in both methods, testing was repeated. Strain H37Ra was used as control. Physicians in charge of TB patients were informed of all the DST results as soon as they were available. These results were then used to modify drug management of the patients accordingly.
Amplification of rpoB, inhA, ahpC and katG genesGenes associated to INH and RIF resistance were amplified as described previously with the following primers: rpoB: 5′-GCGATCAAGGAGTTCTT and biotin labeled 5′-CGATCAGACCGATGTTGG; inhA: biotin labeled 5′-GAGCGTAACCCCAGTGCGAAAG and 5′-CCAGGACTGAACGGGATACGAATG; katG: 5′-GGTCGACATTCGCGAGACGTT and 5′-CGGTGGATCAGCTTGTACCAG; and ahpC: biotin labeled 5′-CGGCACTGCTGAACCACTG and 5′-CCTCATCATCAAAGCGGACAATG.12,21 Briefly, amplifications were performed in a final volume of 50μL that contained 1× PCR buffer, 2mM MgCl2, 0.2mM of each dNTP, 200nM of each primer, 1U of AmpliTaq polymerase (Bioline USA Inc., Randolph, MA, USA) and 200ng of DNA. The thermocycling conditions for rpoB, inhA and ahpC genes were: 94°C for 3min, 45 cycles of 30s at 94°C, 30s at 50°C, and 30s at 72°C, followed by 72°C for 5min.12 The thermocycling conditions for katG gene were: 10 cycles of 15s at 95°C, 1min at 68°C, and 15s at 72°C for 10min, followed by 25 cycles of 15s at 95°C, 15s at 68°C, and 15s at 72°C.21
Pyrosequencing of rpoB, inhA and ahpC genesPCR products of rpoB, inhA and ahpC were then subjected to pyrosequencing analysis, which was performed according to the manufacturer's instructions. Briefly, the biotinylated PCR products were immobilized on streptavidin-coated Sepharose beads and separated by denaturalization with 0.2M NaOH. The single-stranded templates were transferred to a 96-well plate containing annealing buffer and respective sequencing primers.12 The annealing reaction was performed at 90°C for 2min on a thermoblock and pyrosequencing was performed at room temperature on an automated PSQ™ 96 system (Biotage, Inc., Qiagen, Hilden, Germany). The following regions for each gene were sequenced: a 20-bp region of inhA (−24 to −4) with sequencing primer 5′-TGGCAGTCACCCC, a 35-bp region for ahpC (−39 to −4) with sequencing primer 5′-CATTTGGTTGCGACAT and a 57-bp region within the RRDR of rpoB (codon 515–533) with three sequencing reactions, the first reaction sequenced codons 515–526 with sequencing primer 5′-CCAGCTGAGCCAATTC, the second reaction sequenced codons 526–531 with the sequencing primer 5′-TGTCGGGGTTGACC, and the third reaction sequenced codons 531–533 with the sequencing primer 5′-CCACAAGCGCCGA.
PCR-RFLP of katGDue to the inability to pyrosequence the katG product as a result of its size (519bp), digestion with MspA1I of the katG PCR product was performed to generate RFLP patterns for detection of katG315 mutations, as described previously.21 Briefly, ten microliters of restriction enzyme mixture was added to each PCR mixture to give a final volume of 30μL containing a final 0.5U of MspA1I (New England Biolabs, Ipswich, MA, USA), 0.7× New England Biolabs buffer 4 (supplied with enzyme), 100g bovine serum albumin mL−1 according to the manufacturer's instructions. The mixture was incubated at 37°C for 2h in the PCR machine. The products were electrophoresed on a 1.5% agarose gel. A 309-bp band indicated a wild-type katG315 codon. A 377-bp band indicated a mutant type katG315 codon.
Statistical analysisThe Chi-square test was used with Yates’ correction for continuity to compare between resistant and susceptible groups using OpenEpi (Open Source Epidemiologic Statistics for Public Health, Version 2.3.1. www.OpenEpi.com, updated 2011/23/06, accessed 2012/09/25). A p value less than 0.05 was considered significant.
Susceptibility results based on genotypic testing were compared with those of the MGIT and proportion methods. Concordance between proportion and MGIT test results was assessed using the Kappa coefficient (κ). Kappa values indicate weak (≤0.40), moderate (0.41–0.60), strong (0.61–0.80), or excellent (>0.80) agreement. The IBM SPSS statistical software for Windows (SPSS version 20.0; SPSS Inc, Chicago, IL, USA) was used in this analysis.
ResultsPrevalence of M. tuberculosis and drug resistance by both methodsOf the 351 clinical samples processed, 96 (27.4%) were AFB smear-positive specimens and 105 isolates were identified as M. tuberculosis by both biochemical and molecular tests. In addition, 1 (0.9%) isolate was identified as M. bovis and 6 (5.4%) isolates were identified as nontuberculous mycobacteria (NTM) by biochemical and molecular tests.
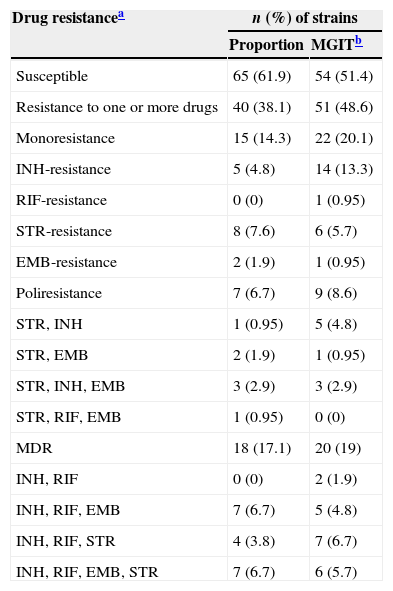
Drug susceptibility data for the four drugs tested are shown in Table 1, where results from proportion and MGIT methods were compared. INH resistance was detected in 42 (40%) isolates (n=28, 26.6% by the proportions method). RIF resistance was detected in 21 (20%) isolates (n=19, 18.1% by the proportions method). MDR was detected in 20 (19%) isolates (n=18, 17.1% by the proportions method). Overall, the MGIT method showed a higher detection level. When comparing methods, RIF and STR resistance results showed excellent concordance (κ=0.969 and κ=0.950, respectively) whereas INH and EMB resistance results showed strong concordance (κ=0.666 and κ=0.744, respectively). In general, SIRE analysis showed strong concordance (κ=0.639).
Phenotypic drug resistance of M. tuberculosis strains.
| Drug resistancea | n (%) of strains | |
|---|---|---|
| Proportion | MGITb | |
| Susceptible | 65 (61.9) | 54 (51.4) |
| Resistance to one or more drugs | 40 (38.1) | 51 (48.6) |
| Monoresistance | 15 (14.3) | 22 (20.1) |
| INH-resistance | 5 (4.8) | 14 (13.3) |
| RIF-resistance | 0 (0) | 1 (0.95) |
| STR-resistance | 8 (7.6) | 6 (5.7) |
| EMB-resistance | 2 (1.9) | 1 (0.95) |
| Poliresistance | 7 (6.7) | 9 (8.6) |
| STR, INH | 1 (0.95) | 5 (4.8) |
| STR, EMB | 2 (1.9) | 1 (0.95) |
| STR, INH, EMB | 3 (2.9) | 3 (2.9) |
| STR, RIF, EMB | 1 (0.95) | 0 (0) |
| MDR | 18 (17.1) | 20 (19) |
| INH, RIF | 0 (0) | 2 (1.9) |
| INH, RIF, EMB | 7 (6.7) | 5 (4.8) |
| INH, RIF, STR | 4 (3.8) | 7 (6.7) |
| INH, RIF, EMB, STR | 7 (6.7) | 6 (5.7) |
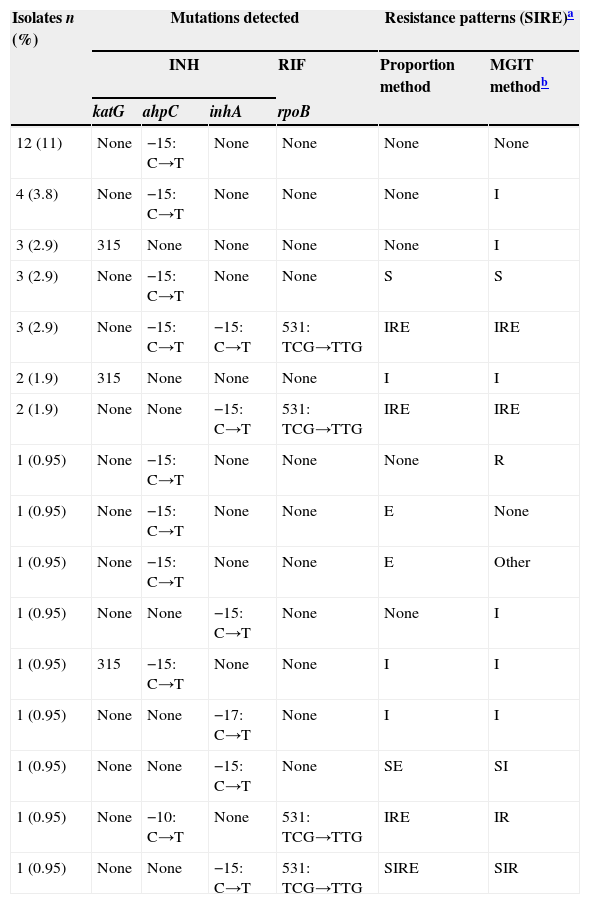
Ser531Leu mutation in rpoB was detected in 10 out of 21 (48%) RIF-resistant isolates (p<0.0001), all MDR by both drug susceptibility testing methods (table 2). Among the 42 INH-resistant isolates (MGIT method), 6 (14%) had a mutation in codon 315 of the katG gene (p<0.05) and 11 (26%) were mutated in the inhA gene (p<0.05): 10 had a −15: C→T substitution and one had a −17:C→T substitution. None of these isolates were mutated in the katG gene; however, 5 had an ahpC mutation and 8 also had mutation in rpoB. All isolates (21%) that had both inhA and rpoB mutations were MDR (Table 2).
Mutations associated to drug resistance detected in M. tuberculosis strains.
| Isolates n (%) | Mutations detected | Resistance patterns (SIRE)a | ||||
|---|---|---|---|---|---|---|
| INH | RIF | Proportion method | MGIT methodb | |||
| katG | ahpC | inhA | rpoB | |||
| 12 (11) | None | −15: C→T | None | None | None | None |
| 4 (3.8) | None | −15: C→T | None | None | None | I |
| 3 (2.9) | 315 | None | None | None | None | I |
| 3 (2.9) | None | −15: C→T | None | None | S | S |
| 3 (2.9) | None | −15: C→T | −15: C→T | 531: TCG→TTG | IRE | IRE |
| 2 (1.9) | 315 | None | None | None | I | I |
| 2 (1.9) | None | None | −15: C→T | 531: TCG→TTG | IRE | IRE |
| 1 (0.95) | None | −15: C→T | None | None | None | R |
| 1 (0.95) | None | −15: C→T | None | None | E | None |
| 1 (0.95) | None | −15: C→T | None | None | E | Other |
| 1 (0.95) | None | None | −15: C→T | None | None | I |
| 1 (0.95) | 315 | −15: C→T | None | None | I | I |
| 1 (0.95) | None | None | −17: C→T | None | I | I |
| 1 (0.95) | None | None | −15: C→T | None | SE | SI |
| 1 (0.95) | None | −10: C→T | None | 531: TCG→TTG | IRE | IR |
| 1 (0.95) | None | None | −15: C→T | 531: TCG→TTG | SIRE | SIR |
Mutations in the ahpC gene were found in both INH susceptible (n=18, 29%) and resistant isolates (n=11, 26%) (p=0.9645). Two site mutations were detected, both at C→T substitutions, situated at positions −15 and −10 in the promoter region.
DiscussionThis is the first analysis of phenotypic resistance to first-line drugs and mutations associated with INH and RIF-resistance of clinical isolates of M. tuberculosis in Guadalajara, Jalisco, Mexico.
When we analyzed proportions and MGIT methods for DST, both showed similar resistance patterns and strong concordance when detecting MDR. The MGIT method showed higher detection levels of INH resistance. In addition, we detected a MDR rate (19%) lower than that previously reported (38%).22rpoB531 is the most frequent mutation among RIF-resistant isolates worldwide23 including Mexico.5–13 Other commonly RIF-resistant associated mutations are rpoB516 and rpoB526.5–7,12,13 Nevertheless, we detected rpoB531 mutation only in half of the RIF-resistant isolates. The use of a 57-bp region rather than the entire 81-bp hotspot of rpoB may explain this relatively low proportion of RIF-resistant strains with rpoB mutations in the current study. In addition, mutations outside RRDR have also been found in RIF-resistant isolates worldwide,23 including Mexico6,8,10; therefore, some of the RIF-resistant isolates that did not show a mutation may have a mutation in these regions. Further analysis of the whole RRDR region of these isolates may help detect other mutations.
It has been demonstrated that almost all RIF-resistant strains also show resistance to other drugs, mainly to INH.24 In fact, RIF-resistant detection has been proposed as an early indicator of MDR-TB. Our results show that almost all (95%) RIF-resistant isolates analyzed were also INH-resistant. To delineate such role, we need to run more clinical specimens to define if the rpoB gene could be used as a surrogate marker to early detect MDR-TB.
An unusually low frequency of katG mutations (14%) in INH-resistant isolates was found in our study. Although it has been reported that katG315 mutation can account for less than a quarter of katG mutations in INH resistant strains,25 mutations at different sites other than the 315 codon of the katG gene may be present in our INH resistant isolates.9–11,14 Other strategies such as complete sequencing of katG may be helpful in defining the mutations associated to INH resistance.
As expected, we found a frequency of mutations (26%) in the promoter region of inhA similar to data reported before, which is 20–34% of INH-resistant strains.3,4 To the best of our knowledge, only three other studies have reported inhA mutations in Mexico, all of which shared the same mutation we found.9,12,13
In our study, both INH susceptible and resistant isolates were found to be mutated in the ahpC gene. Although initially ahpC was associated to INH resistance,26 reports of ahpC promoter mutations together with katG deletion indicate an increase in the expression of ahpC as a compensatory mutation for the loss of catalase/peroxidase activity rather than the cause of INH resistance directly.4,27 Additionally, INH susceptible strains harboring an ahpC mutation have been reported before, in positions such as −32 and −10.12,28 Consequently, this sequence alteration has been suggested to actually be a polymorphism.28 Considering we did not have deletion of katG, our results strongly support this idea.
Almost half of INH-resistant isolates (48%) in our study did not have any mutation at all. Thus, further research is needed to expand the analysis of regions of both katG and inhA, in search of additional mutations.
In conclusion, this is the first analysis of mutations associated with INH and RIF-resistance of clinical isolates of M. tuberculosis in Guadalajara, Jalisco, Mexico, where most commonly reported mutations worldwide were detected. Additionally, fingerprinting (IS6110-RFLP and spoligotyping) patterns analysis would determine if these isolates belong in the same cluster. The evaluation of different DR profiles and resistance-associated mutations in the same cluster could determine whether mutations were acquired independently, or whether there is a presence and dissemination of a successful clone in the Guadalajara community; thus the analysis of clone polymorphisms in this population is imperative. This is an ongoing research by our group.
Conflict of interestThe authors declare no conflict of interest.