There are not data on blood B-cell lymphoma 2 (Bcl-2) concentrations (one of the antiapoptotic molecules of the Bcl-2 family in the intrinsic apoptosis pathway) in septic patients. Therefore, this study was carried with the aims to explore whether blood Bcl-2 concentrations at diagnosis of sepsis are different in survivor and non-survivor septic patients, are associated with mortality, and are useful for the mortality prediction.
MethodsIntensive Care Units from 3 Spanish hospitals participated in this observational and prospective study with septic patients and serum Bcl-2 concentrations at diagnosis of sepsis were determined. Mortality at 30 days was as outcome variable.
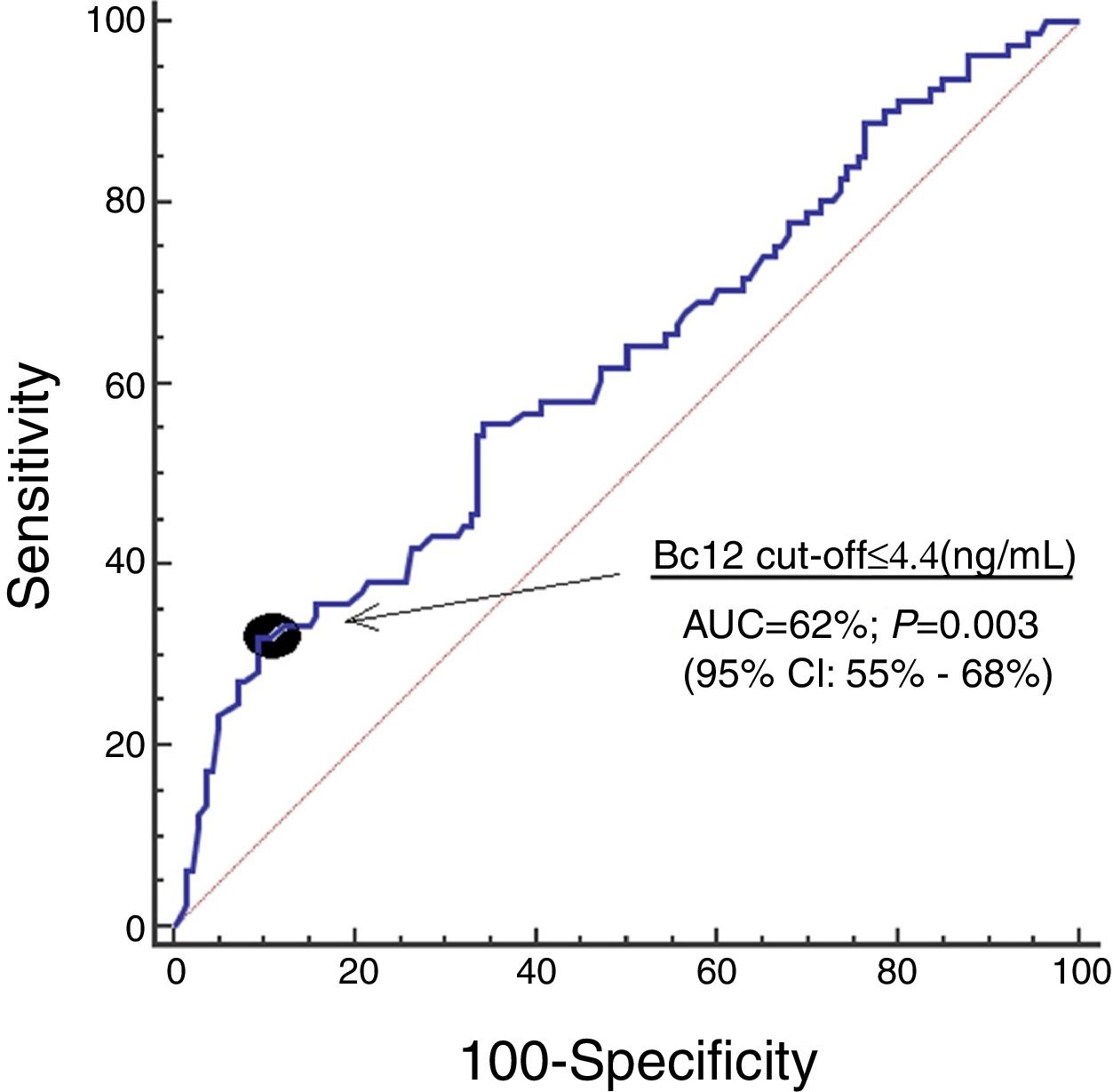
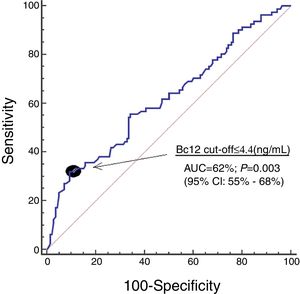
ResultsWe found that 30-day non-surviving patients (n=81) showed lower serum Bcl-2 levels (p=0.003) than surviving patients (n=140). We found that serum concentrations of Bcl-2<4.4ng/mL were associated with mortality (OR=3.228; 95% CI=1.406–7.415; p=0.006) in the multiple logistic regression analysis, and that showed an area under the curve for mortality prediction of 62% (95% CI=55–68%; p=0.003).
ConclusionsIn our study appears novel findings such as higher blood Bcl-2 concentrations in survivor than in non-survivor septic patients, the association between low blood Bcl-2 concentrations and mortality of septic patients, and the ability of blood Bcl-2 concentrations for the prediction of septic patient mortality.
No hemos encontrado datos publicados sobre las concentraciones sanguíneas de B-cell lymphoma 2 (Bcl-2) (una de las moléculas antiapoptóticas de la familia Bcl-2 de la vía intrínseca de la apoptosis) en pacientes con sepsis. Por lo cual, este estudio se realizó con los propósitos de explorar las concentraciones sanguíneas de Bcl-2 en el momento del diagnóstico de la sepsis en los pacientes supervivientes y fallecidos, analizar si se asocian con la supervivencia y determinar si son útiles para predecir el fallecimiento.
MétodosLas unidades de Cuidados Intensivos de 3 hospitales españoles participaron en este estudio observacional y prospectivo de pacientes con sepsis, y se determinaron las concentraciones sanguíneas de Bcl-2 al diagnosticar la sepsis. La mortalidad a 30 días fue nuestra variable resultado.
ResultadosLos pacientes que fallecían en los primeros 30 días (n=81) presentaron menores concentraciones sanguíneas de Bcl-2 (p=0,003) que los supervivientes (n=140). Encontramos que las concentraciones sanguíneas de Bcl-2<4,4 ng/ml se asociaban con la mortalidad en el análisis de regresión logística múltiple (OR=3,228; IC del 95%=1,406-7,415; p=0,006). Encontramos que las concentraciones sanguíneas de Bcl-2 tenían un área bajo la curva del 62% (IC del 95%=55%-68%; p=0,003) para la predicción del fallecimiento.
ConclusionesEn nuestro estudio aparecieron nuevos hallazgos como que las concentraciones sanguíneas de Bcl-2 fueron superiores en los supervivientes, se asociaban con la supervivencia y podían predecir la mortalidad.
Sepsis produced a great number of deaths and consumption of healthcare resources.1,2 Apoptosis, which is the process by which the cell death by a programmed and actively way, appears in some physiological processes (morphogenesis and tissue remodelling) and in some diseases.3–5 In fact, there has been found an increase of apoptosis in sepsis animal models.3–5 The two main pathways of cell death by apoptosis are the extrinsic (or death receptor pathway) and the intrinsic (or mitochondrial pathway).
The intrinsic pathway can be induced by several stressors such as oxidative stress, proinflammatory cytokines, genetic mutations, deficits of growth factors, and excitotoxicity, which opens the mitochondrial transition pores (MTP) allowing the transportation of cytochrome c from the mitochondria into the cytosol where binds with the apoptotic protease activating factor (Apaf)-1 and procaspase-9 forming an apoptosome that is involving in the activation of caspase-9 (initiator caspase) that activates executor caspases (caspases 3 and 7) that produce the cellular apoptotic changes.3–5 There are pro-apoptotic B-cell lymphoma-2 (Bcl-2) family members (such as Bax, Bak, Bad, Bim, Bid) that promote the MTP formation and the release of cytochrome into the cytosol, and anti-apoptotic members of the Bcl-2 family (Bcl-2, Bcl-XL, Bclw) that block the MTP formation and the release of cytochrome c.3–5
Decreased expression of Bcl-2 (one member of the anti-apoptotic Bcl-2 family) in white blood cells in septic patients than in control subjects have been found6–10 and in non-survivor than in survivor septic patients has been found.10 Circulating Bcl-2 concentrations have been previously reported in patients with different diseases.11–15 However, there are not data on circulating Bcl-2 concentrations in septic patients. Therefore, this study was carried with the aims to explore whether blood Bcl-2 concentrations are different in survivor and non-survivor septic patients, are associated with mortality, and are useful for the mortality prediction.
Material and methodsDesign and subjectsIntensive Care Units from 3 Spanish hospitals participated in this prospective and observational study with septic patients. The study was conducted with the approval in all hospitals of the Ethics Committee. We obtained, for the participation in the study, the informed and signed consent from patients or their relatives. The study was conducted between 2013 and 2014.
We included septic patients and sepsis was defined using Sepsis-3 Consensus criteria.16 We excluded the patients with blood cell count<1000/μl, hematological tumor, human immunodeficiency virus (HIV), white solid tumor, breastfeeding, pregnancy, age<18 years immunosuppressive therapy, radiation therapy, or steroid agents.
We collect different epidemiological and clinical variables at moment of sepsis diagnosis. Sex, age, and the history of chronic obstructive pulmonary disease, ischemic heart disease, chronic renal failure, and diabetes mellitus were registered. We also recollected international normalized ratio, platelets, activated partial thromboplastin time, pressure of arterial oxygen, fraction inspired of oxygen, creatinine, leukocytes, bilirubin, lactic acid, Acute Physiology and Chronic Health Evaluation (APACHE)-II score,17 Sepsis-related Organ Failure Assessment [SOFA] score18 and site of infection. In addition, survival at 30 days (our endpoint study), and the appearance of bloodstream infection and septic shock were recorded.
Determination of serum Bcl-2 concentrationsWe collected serum of patient at moment of sepsis diagnosis and was frozen at −80°C until the moment of concentration determination. We used the Human Bcl-2 ELISA Kit (Elabscience, Houston, Texas, United States) for the determination of serum Bcl-2 concentrations. The detection limit of the assay was 0.10ng/mL, the intra-assay and inter-assay coefficients of variation were equal or lower to 6%.
Statistical methodsWe used frequencies (percentages) and medians (percentile 25–75) to describe categorical and continuous variables. We used chi-square test and Mann–Whitney U test to compare categorical and continuous variables between patient groups (surviving and non-surviving). To test the ability of serum Bcl-2 concentrations for mortality prediction was performed a receiver operating characteristic analysis. We used the Youden J index to construct Kaplan–Meier 30-day survival curves, and therefore serum Bcl-2 concentration of 4.4ng/mL was the point level used. The possible association between serum Bcl-2 concentrations and 30-day mortality was tested by a multiple logistic regression analysis. We used the point p<0.05 for the establishment of significant differences, and the programs NCSS 2000 (Kaysville, Utah) and SPSS 17.0 (SPSS Inc., Chicago, IL, USA) for the analyses.
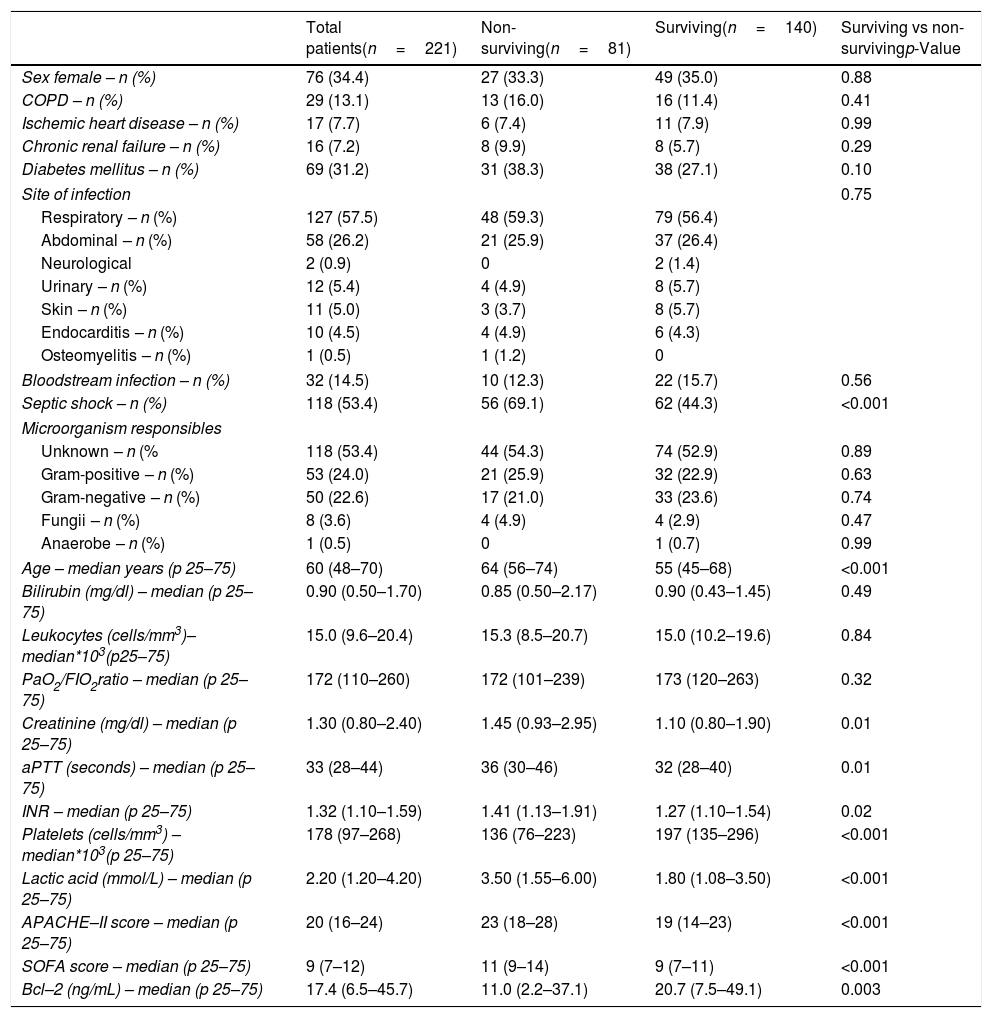
ResultsWe found that non-surviving patients (n=81) showed higher SOFA, age, rate of septic shock and serum lactic acid levels than surviving patients (n=140), and lower serum levels of Bcl-2 (p=0.003) (Table 1). The number of patients included in each hospital were 121, 68 and 32 patients respectively, and there were not differences in the mortality rate (p=0.67).
Demographic and clinical characteristics of non-surviving and surviving septic patients.
| Total patients(n=221) | Non-surviving(n=81) | Surviving(n=140) | Surviving vs non-survivingp-Value | |
|---|---|---|---|---|
| Sex female – n (%) | 76 (34.4) | 27 (33.3) | 49 (35.0) | 0.88 |
| COPD – n (%) | 29 (13.1) | 13 (16.0) | 16 (11.4) | 0.41 |
| Ischemic heart disease – n (%) | 17 (7.7) | 6 (7.4) | 11 (7.9) | 0.99 |
| Chronic renal failure – n (%) | 16 (7.2) | 8 (9.9) | 8 (5.7) | 0.29 |
| Diabetes mellitus – n (%) | 69 (31.2) | 31 (38.3) | 38 (27.1) | 0.10 |
| Site of infection | 0.75 | |||
| Respiratory – n (%) | 127 (57.5) | 48 (59.3) | 79 (56.4) | |
| Abdominal – n (%) | 58 (26.2) | 21 (25.9) | 37 (26.4) | |
| Neurological | 2 (0.9) | 0 | 2 (1.4) | |
| Urinary – n (%) | 12 (5.4) | 4 (4.9) | 8 (5.7) | |
| Skin – n (%) | 11 (5.0) | 3 (3.7) | 8 (5.7) | |
| Endocarditis – n (%) | 10 (4.5) | 4 (4.9) | 6 (4.3) | |
| Osteomyelitis – n (%) | 1 (0.5) | 1 (1.2) | 0 | |
| Bloodstream infection – n (%) | 32 (14.5) | 10 (12.3) | 22 (15.7) | 0.56 |
| Septic shock – n (%) | 118 (53.4) | 56 (69.1) | 62 (44.3) | <0.001 |
| Microorganism responsibles | ||||
| Unknown – n (% | 118 (53.4) | 44 (54.3) | 74 (52.9) | 0.89 |
| Gram-positive – n (%) | 53 (24.0) | 21 (25.9) | 32 (22.9) | 0.63 |
| Gram-negative – n (%) | 50 (22.6) | 17 (21.0) | 33 (23.6) | 0.74 |
| Fungii – n (%) | 8 (3.6) | 4 (4.9) | 4 (2.9) | 0.47 |
| Anaerobe – n (%) | 1 (0.5) | 0 | 1 (0.7) | 0.99 |
| Age – median years (p 25–75) | 60 (48–70) | 64 (56–74) | 55 (45–68) | <0.001 |
| Bilirubin (mg/dl) – median (p 25–75) | 0.90 (0.50–1.70) | 0.85 (0.50–2.17) | 0.90 (0.43–1.45) | 0.49 |
| Leukocytes (cells/mm3)–median*103(p25–75) | 15.0 (9.6–20.4) | 15.3 (8.5–20.7) | 15.0 (10.2–19.6) | 0.84 |
| PaO2/FIO2ratio – median (p 25–75) | 172 (110–260) | 172 (101–239) | 173 (120–263) | 0.32 |
| Creatinine (mg/dl) – median (p 25–75) | 1.30 (0.80–2.40) | 1.45 (0.93–2.95) | 1.10 (0.80–1.90) | 0.01 |
| aPTT (seconds) – median (p 25–75) | 33 (28–44) | 36 (30–46) | 32 (28–40) | 0.01 |
| INR – median (p 25–75) | 1.32 (1.10–1.59) | 1.41 (1.13–1.91) | 1.27 (1.10–1.54) | 0.02 |
| Platelets (cells/mm3) – median*103(p 25–75) | 178 (97–268) | 136 (76–223) | 197 (135–296) | <0.001 |
| Lactic acid (mmol/L) – median (p 25–75) | 2.20 (1.20–4.20) | 3.50 (1.55–6.00) | 1.80 (1.08–3.50) | <0.001 |
| APACHE–II score – median (p 25–75) | 20 (16–24) | 23 (18–28) | 19 (14–23) | <0.001 |
| SOFA score – median (p 25–75) | 9 (7–12) | 11 (9–14) | 9 (7–11) | <0.001 |
| Bcl–2 (ng/mL) – median (p 25–75) | 17.4 (6.5–45.7) | 11.0 (2.2–37.1) | 20.7 (7.5–49.1) | 0.003 |
COPD=Chronic Obstructive Pulmonary Disease; PaO2/FIO2=pressure of arterial oxygen/fraction inspired oxygen; aPTT=Activated partial thromboplastin time; INR=International normalized ratio; APACHE=Acute Physiology and Chronic Health Evaluation; SOFA=Sepsis-related Organ Failure Assessment.
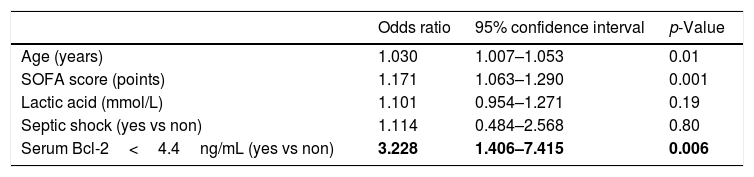
We found that serum concentrations of Bcl-2<4.4ng/mL were associated with mortality (OR=3.228; 95% CI=1.406–7.415; p=0.006) after controlling for age, SOFA, lactic acid and septic shock in the multiple logistic regression analysis (Table 2).
Multiple logistic regression analyses to predict mortality at 30 days.
| Odds ratio | 95% confidence interval | p-Value | |
|---|---|---|---|
| Age (years) | 1.030 | 1.007–1.053 | 0.01 |
| SOFA score (points) | 1.171 | 1.063–1.290 | 0.001 |
| Lactic acid (mmol/L) | 1.101 | 0.954–1.271 | 0.19 |
| Septic shock (yes vs non) | 1.114 | 0.484–2.568 | 0.80 |
| Serum Bcl-2<4.4ng/mL (yes vs non) | 3.228 | 1.406–7.415 | 0.006 |
SOFA=Sepsis-related Organ Failure Assessment.
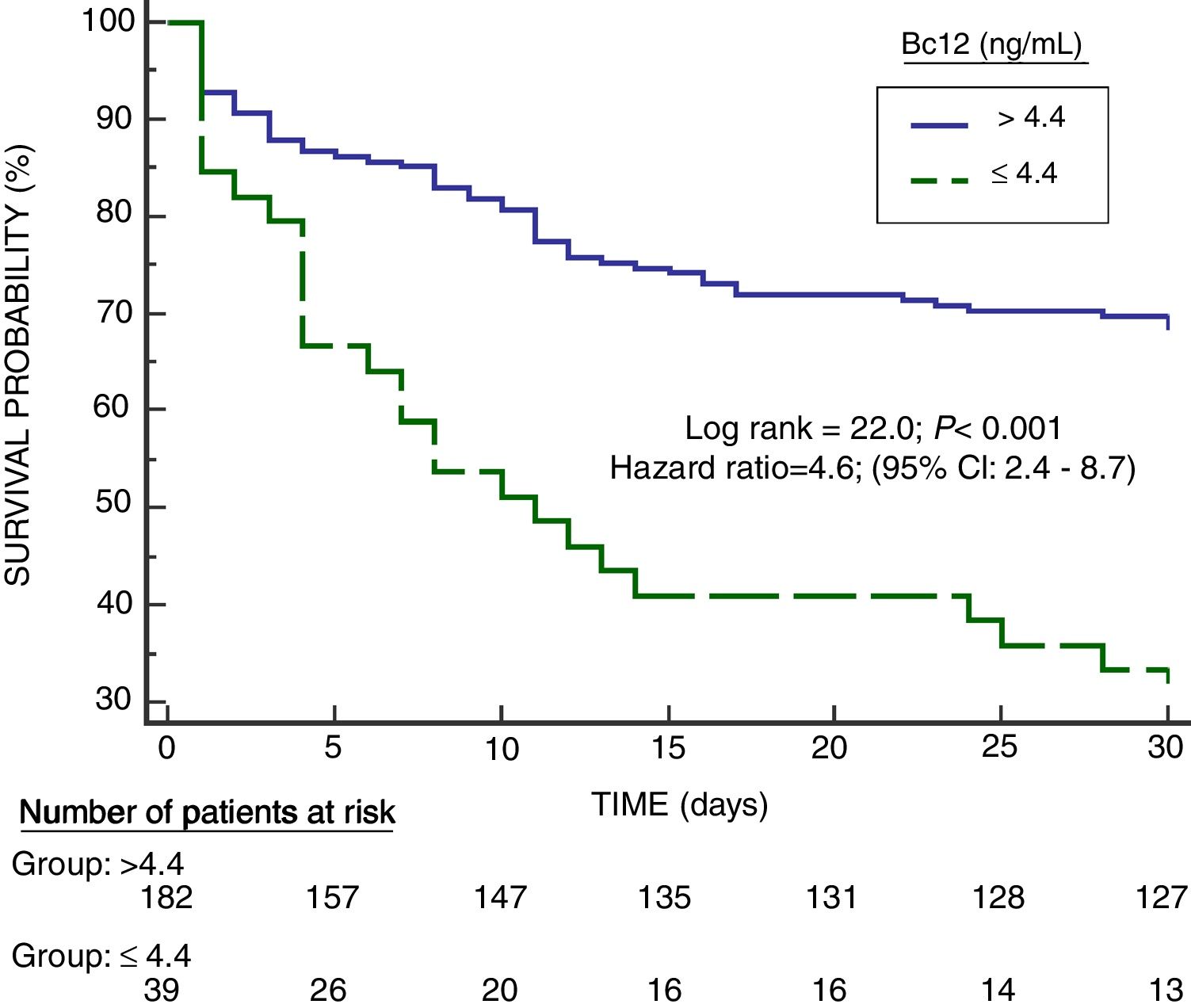
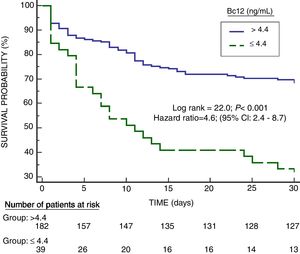
Serum Bcl-2 concentrations showed an area under the curve for mortality prediction of 62% (95% CI=55–68%; p=0.003) (Fig. 1). A higher mortality rate (Hazard ratio=4.6; 95% CI=2.4–8.7; p<0.001) was found in patients with serum Bcl-2 levels lower or equal to 4.4ng/mL in Kaplan–Meier analysis (Fig. 2).
We no found significant differences in serum Bcl-2 levels regarding to site of infection (p=0.22), the presence or not of bloodstream infection (p=0.27), and that the microorganism responsible was Gram-positive (p=0.70), Gram-negative (p=0.43), fungii (p=0.61), anaerobe (p=0.15) or unknown (p=0.28).
DiscussionPreviously, there were found lower expression of Bcl-2 in white blood cells in septic patients than in control subjects6–10 and in non-survivor than in survivor septic patients.10 Thus, to the best of our knowledge, this is the first study reporting data on blood Bcl-2 concentrations in septic patients. In our study appears novel findings such as higher blood Bcl-2 concentrations in survivor than in non-survivor patients, the association between low blood Bcl-2 concentrations and mortality of septic patients, and the ability of blood Bcl-2 concentrations for the prediction of septic patient mortality.
Bcl-2 is a member of the anti-apoptotic Bcl-2 family that block the mitochondrial transition pores formation and therefore difficult the release of cytochrome c from the mitochondria into the cytosol.3–5 Therefore, Bcl-2 block the formation of the apoptosma between cytochrome c, Apaf-1 and procaspase-9 and therefore the activation of caspase-9. Thus, Bcl-2 block the activation of executor caspases (caspases 3 and 7).3–5 Therefore, we think, that the higher mortality in septic patients with low blood Bcl-2 concentrations found in our study could be due to that non-surviving patients have a lower block of mitochondrial transition pores formation leading to a higher activation of apoptosis intrinnsic pathway, which produces a higher activation of effector caspases and cellular damage by apoptosis. However, we must recognize some limitations of our study. First, we have not determined blood concentrations of other anti-apoptotic members of the Bcl-2 family (such as Bcl-XL, Bclw) and of pro-apoptotic Bcl-2 family members (such as Bax, Bak, Bad, Bim, Bid). Second, we have not assessed cellular damage by apoptosis. Third, the AUC of serum Bcl-2 concentrations for mortality prediction was statistically significant; however, this AUC was not high. Fourth, the percentage of excluded patients was not registered. Fifth, the rate of bloodstream infection was lower than in other series reaching 40%.19 Another interesting point is that in animals models the use of adrenomedullin in septic rats has been associated with an increase of Bcl-2 expression and a decrease of apoptosis.20 Therefore, could be interesting to research about the role of blood Bcl-2 concentrations as prognostic biomarker in septic patient due to that its determination is easy and cheap and about the use of agents that increase Bcl-2 for the treatment of sepsis.
ConclusionsIn conclusion, to the best of our knowledge, this is the first study reporting data on blood Bcl-2 concentrations in septic patients. In our study, appears novel findings such as higher blood Bcl-2 concentrations in survivor than in non-survivor patients, the association between low blood Bcl-2 concentrations and mortality of septic patients, and the ability of blood Bcl-2 concentrations for the prediction of septic patient mortality.
FundingThis study was supported by a grant from Instituto de Salud Carlos III (PI-18-00500) (Madrid, Spain) and co-financed with Fondo Europeo de Desarrollo Regional (FEDER).
Conflict of interestThe authors declare that they have no competing interests.