Currently, the microbiological diagnosis of genital infections is carried out with molecular methods, which allow the detection of less frequent etiological agents but with potential pathogenic importance, such as Haemophilus spp. The objective of this review is to analyse and highlight the clinical importance of the isolation of Haemophilus spp. in genital and rectal infections, excluding Haemophilus ducreyi.
Material and methodsA systematic review was carried out based on an exhaustive search of the publications included in the MEDLINE database up to August 5, 2021, on the presence of Haemophilus spp. in genital and rectal infections, excluding H. ducreyi.
ResultsAfter reviewing what was described in the literature, Haemophilus spp. (excluding H. ducreyi: HSNOD) was detected in 2397 episodes of genital infection, the most frequently isolated species being H. influenzae and H. parainfluenzae. Most of the episodes (87,6%) are constituted by single isolation. There is a slight predominance in women (48,3%) where it can cause vaginitis, salpingitis, endometritis or complications during pregnancy. In men, the clinical picture usually corresponds to urethritis. Most of the samples correspond to vaginal and urethral exudates, with a minority representation at the rectal level (2.3%).
ConclusionHSNOD plays a relevant pathogenic role in episodes of genital infection, so microbiological diagnostic protocols must include methods that allow their detection, as well as include them in the etiological spectrum of this type of clinical picture.
Actualmente el diagnóstico microbiológico de las infecciones genitales se realiza con métodos moleculares, los cuales permiten detectar agentes etiológicos menos frecuentes, pero con potencial importancia patogénica, como Haemophilus spp. El objetivo de esta revisión es analizar y resaltar la importancia clínica del aislamiento de Haemophilus spp. en infecciones genitales y rectales, excluyendo H. ducreyi.
Material y métodosSe ha realizado una revisión sistemática en base a una búsqueda exhaustiva de las publicaciones incluidas en la base de datos MEDLINE hasta el 5 de agosto de 2021, sobre la presencia de Haemophilus spp. en infecciones genitales y rectales, excluyendo H. ducreyi.
ResultadosTras revisar lo descrito en la literatura, las especies de Haemophilus (excluyendo H. ducreyi: HSNOD) se detectaron en 2397 episodios de infección genital, siendo las especies más frecuentemente aisladas H. influenzae y H. parainfluenzae. La mayoría de los episodios (87,6%) están constituidos por aislamiento único. Existe un ligero predominio en mujeres (48,3%) donde puede producir cuadros de vaginitis, salpingitis, endometritis o complicaciones durante el embarazo. En hombres, el cuadro clínico suele corresponder a una uretritis. La mayoría de las muestras corresponde a exudados vaginales y uretrales, con una representación minoritaria a nivel rectal (2,3%).
ConclusiónHSNOD desempeña un papel patogénico relevante en episodios de infección genital, por lo que los protocolos de diagnóstico microbiológico deben incluir métodos que permitan su detección, así como incluirlos en el espectro etiológico de este tipo de cuadros clínicos.
Genital tract infections are common entities that share a clinical presentation but can be caused by myriad aetiological agents apart from the microorganisms commonly regarded as pathogens1. In addition, there is a large diversity of microbiota and significant inter-individual variability that makes it even more difficult to distinguish between a possible pathogenic role or not2. These less common agents that play a possibly pathogenic role include the species of the genus Haemophilus, excluding Haemophilus ducreyi (HSNOTD). The genus Haemophilus, belonging to the Pasteurallaceae family, is made up of small non-sporulating, pleomorphic, non-motile, facultative anaerobic gram-negative bacilli. It is characterised by having demanding nutritional requirements and requires chocolate agar medium for proper growth and identification. The species of HSNOTD most frequently involved are Haemophilus influenzae (H. influenzae), Haemophilus parainfluenzae (H. parainfluenzae) and Haemophilus haemolyticus (H. haemolyticus). The first one is well-known, it is a colonising agent of the upper respiratory and genital tract and can behave as an opportunistic pathogen. In recent years, more and more cases of urethritis associated with H. influenzae, and more recently its association with non-gonococcal urethritis, have been described both in men who have sex with men (MSM) and women3. In addition, it is the second most common cause of vulvovaginitis in girls not linked to sexual transmission4. H. parainfluenzae predominantly colonises the oropharynx and can lead to infections through contiguity, bacteraemia, endocarditis and genital infections5. H. haemolyticus is difficult to distinguish from the non-encapsulated species of H. influenzae due to its morphological, biochemical and genetic similarities, and it is sometimes difficult to distinguish between them by conventional microbiological methods, hence some authors advocate the combination of conventional methods and polymerase chain reaction (PCR) analysis; 6 mass spectrometry (MALDI-TOF) has also shown good results in differentiation capacity7. It rarely has pathogenic potential.
In addition, there are the biotype IV cryptic genospecies, genetically related to H. haemolyticus, but with a different phenotype and location, such as "Haemophilus quentini", whose presence has been described in the genitourinary tract and can cause genital infections in pregnant women, chorioamnionitis and preterm delivery, and even pneumonic or septic conditions in newborns8.
The objective of this study is to analyse the presence of HSNOTD species in the development of genital infections through a systematic literature review.
Materials and methodsA search was made in the MEDLINE database, through PubMed, for studies that describe the presence of Haemophilus spp. in genital (vaginal, endocervical and urethral) and rectal exudates (in MSM). The following search terms were used: "Haemophilus and urethritis", "Haemophilus and proctitis", "Haemophilus and vaginitis", "Haemophilus and cervicitis", "Haemophilus and salpingitis", "Haemophilus and endometritis", "Haemophilus and bartholinitis", "Haemophilus and tubo-ovarian abscess", "Haemophilus and septic abortion", "Haemophilus and chorionamnionitis". The inclusion criteria were: studies published up until 1 September 2021; and studies published in English or Spanish. The exclusion criteria were: studies referring to the H. ducreyi or Haemophilus vaginalis species; and studies that analysed samples from sources other than genital or rectal. The references listed in the studies were also reviewed to reduce the number of losses.
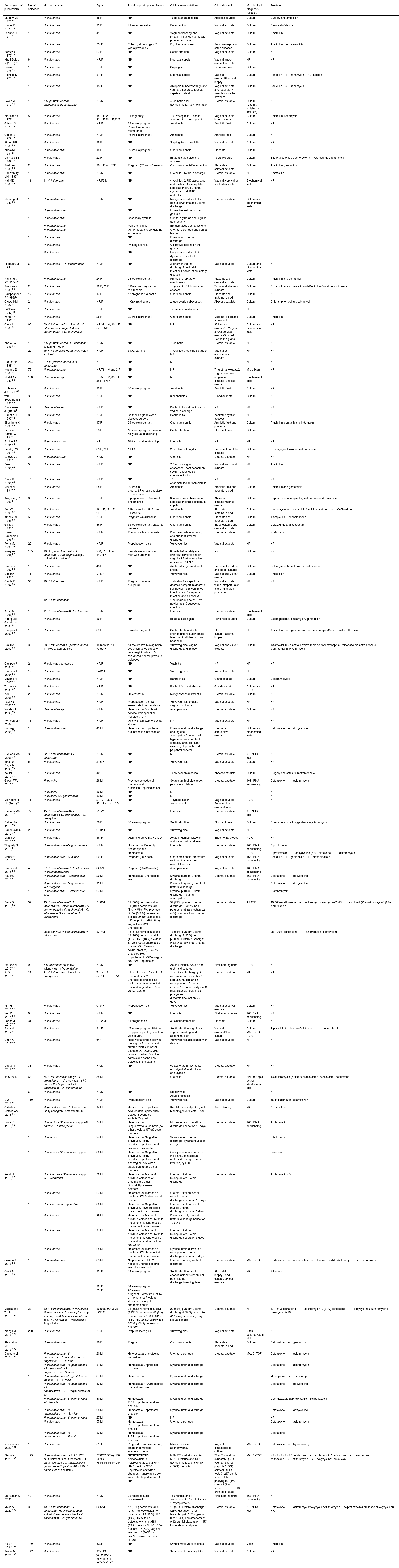
Results145, 3, 583, 595, 21, 73, 14, 6, 16 and 30 publications were obtained for the criteria "Haemophilusand urethritis", "Haemophilusand proctitis", "Haemophilusand vaginitis", "Haemophilusand cervicitis", "Haemophilusand salpingitis", "Haemophilusand endometritis", "Haemophilusand bartholinitis", "Haemophilusand tubo-ovarian abscess", "Haemophilusand septic abortion", "Haemophilus and chorionamnionitis", respectively, which were subsequently submitted to the inclusion and exclusion criteria, yielding 117 studies. Of these, 13 could not be located (Northwest Med. 1955;54(9):992−3, J Pathol Bacteriol. 1967;93(1):109−18, Am J Vet Res. 1971;32(12):2067−9, Am J Clin Pathol. 1980;73(2):285−7, Med J Aust. 1985;143(5):223, Med J Aust. 1985;142(9):531, J Infect Dis. 1986;153(1):165−7, Acta Paediatr Scand. 1987;76(2):363−4, Med Clin (Barc). 1989;92(9):335−7, Med Clin (Barc). 1989;93(19):758−9, Pediatr Infect Dis J. 1994;13(3):243, Am J Obstet Gynecol. 1994;170(4):1008−17, J Am Board Fam Pract. 1994;7(4):335−41). Table 1 shows 104 works, which group together a total of 2397 episodes of genital infection caused by Haemophilus spp. The most frequently isolated species were H. influenzae (57.7%; 1383/2397) and H. parainfluenzae (35.7%; 855/2397). No species was identified in 6.7% (159/2397) of the episodes. In most episodes (87.6%; 2099/2397), Haemophilus was isolated in monomicrobial culture. Otherwise, it was often detected along with Chlamydia trachomatis, Neisseria gonorrhoeae, Ureaplasma spp. or Mycoplasma spp.
Studies reporting the isolation of Haemophilus spp. in genital samples.
| Author (year of publication) | No. of episodes | Microorganisms | Age/sex | Possible predisposing factors | Clinical manifestations | Clinical sample | Microbiological diagnosis reflected | Treatment |
|---|---|---|---|---|---|---|---|---|
| Skirrow MB (1970)9 | 1 | H. influenzae | 48/F | NP | Tubo-ovarian abscess | Abscess exudate | Culture | Surgery and ampicillin |
| Hurley R (1970)10 | 1 | H. influenzae | 29/F | Intrauterine device | Endometritis | Vaginal exudate | Culture | Removal of device |
| Farrand RJ (1971)11 | 1 | H. influenzae | 4/ F | NP | Vaginal dischargeand irritation Inflamed vagina with purulent exudate | Vaginal exudate | Culture | Ampicillin |
| 1 | H. influenzae | 35/ F | Tubal ligation surgery 7 years previously. | Right tubal abscess | Puncture-aspiration of the abscess | Culture | Ampicillin+cloxacillin | |
| Berczy J (1973)12 | 1 | H. influenzae | 27/F | NP | Septic abortion | Vaginal exudate | Culture | NP |
| Khuri-Bulos N (1975)13 | 8 | H. influenzae | NP/F | NP | Neonatal sepsis | Vaginal and/or cervical exudate | NP | NP |
| Herva E (1975)14 | 1 | H. influenzae | NP/F | NP | Salpingitis | Tubal exudate | Culture | NP |
| Nicholls S (1975)15 | 1 | H. influenzae | 31/ F | NP | Neonatal sepsis | Vaginal exudatePlacental biopsy | Culture | Penicillin+kanamycin (NR)Ampicillin |
| 1 | H. influenzae | 18/ F | NP | Antepartum haemorrhage and vaginal discharge.Neonatal sepsis and death | Vaginal exudate and respiratory samples from the newborn | Culture | Penicillin+kanamycin | |
| Bowie WR (1977)16 | 10 | 7 H. parainfluenzae4 + C. trachomatis3 H. influenzae | NP/M | NP | 4 urethritis and3 asymptomatic3 asymptomatic | Urethral exudate | Culture (Virginia Polytechnic Institute) | NP |
| Albritton WL (1978)17 | 5 | H. influenzae | 18F, 20F, 22F 30F,33/F | 2 Pregnancy | 1 vulvovaginitis, 2 septic abortion, 1 acute salpingitis | Vaginal exudate, blood cultures | Culture | Ampicillin, kanamycin |
| Gibson M (1978)18 | 1 | H. influenzae | NP/F | 28 weeks pregnant. Premature rupture of membranes | Amnionitis | Amniotic fluid | Culture | NP |
| Ogden E (1979)19 | 1 | H. influenzae | NP/F | 16 weeks pregnant | Amnionitis | Amniotic fluid | Culture | NP |
| Simon HB (1980)20 | 1 | H. influenzae | 36/F | NP | Salpingitis/endometritis | Vaginal exudate | Culture | NP |
| Arias JW (1981)21 | 1 | H. parainfluenzae | 18/F | 29 weeks pregnant | Chorioamnionitis | Placenta | Culture | NP |
| De Pass EE (1982)22 | 1 | H. influenzae | 22/F | NP | Bilateral salpingitis and abscess | Tubal exudate | Culture | Bilateral salpingo-oophorectomy, hysterectomy and ampicillin |
| Pastorek J (1982)23 | 2 | H. influenzae | 26F and 17F | Pregnant (37 and 40 weeks) | ChorioamnionitisEndometritis | Placenta and cervical exudate | Culture | Ampicillin, gentamicin |
| Chowdhury MN (1983)24 | 1 | H. parainfluenzae | NP/M | NP | Urethritis, urethral discharge | Urethral exudate | NP | Amoxicillin |
| Hall GD (1983)25 | 11 | 11 H. influenzae | NP/F2 M | NP | 4 vaginitis, 2 IUD-associated endometritis, 1 incomplete septic abortion, 1 urethral syndrome and 1NP2 urethritis | Vaginal, cervical or urethral exudate | Biochemical tests | NP |
| Messing M (1983)26 | 1 | H. parainfluenzae | NP/M | NP | Nongonococcal urethritis: genital erythema and urethral discharge | Urethral exudate | Culture and biochemical tests | NP |
| 1 | H. parainfluenzae | NP | Ulcerative lesions on the genitals | |||||
| 1 | H. parainfluenzae | Secondary syphilis | Genital erythema and inguinal adenopathy | |||||
| 1 | H. parainfluenzae | Pubic folliculitis | Erythematous genital lesions | |||||
| 1 | H. parainfluenzae | Gonorrhoea and condyloma acuminata | Urethral discharge and genital lesion | |||||
| 1 | H. influenzae | NP | Dysuria and urethral discharge | |||||
| 1 | H. influenzae | Primary syphilis | Ulcerative lesions on the genitals | |||||
| 1 | H. influenzae | NP | Nongonococcal urethritis: dysuria and urethral discharge | |||||
| Tebbutt GM (1984)27 | 6 | H. influenzae1 + N. gonorrhoeae | NP/F | NP | 3 girls with vaginal discharge2 postnatal infection1 pelvic inflammatory disease | Vaginal exudate | Culture and biochemical tests | NP |
| Nakamura KT (1984)28 | 1 | H. parainfluenzae | 24/F | 28 weeks pregnant. | Premature rupture of membranes | Placenta and cervical exudate | Culture | Ampicillin and gentamicin |
| Paavonen J (1985)29 | 2 | H. influenzae | 22/F, 29/F | 1 Previous risky sexual relationship | 1 pyosalpinx1 tubo-ovarian abscess | Tubal and abscess exudate | Culture | Doxycycline and metronidazolePenicillin G and metronidazole |
| Campognone P (1986)30 | 17 | H. influenzae | 17 F | 17 pregnant. 1 diabetic | Chorioamnionitis | Placenta and maternal blood | Culture | NP |
| Crowe HM (1987)31 | 2 | H. influenzae | NP/F | 1 Crohn's disease | 2 tubo-ovarian abscesses | Abscess exudate | Culture | Chloramphenicol and tobramycin |
| LW Davis (1987) 32 | 1 | H. influenzae | NP/F | NP | Tubo-ovarian abscess | NP | NP | NP |
| Winn HN (1987)33 | 1 | H. influenzae | 25/F | 22 weeks pregnant. | Chorioamnionitis | Maternal blood and amniotic fluid | Culture | Ampicillin |
| Casin I (1988)34 | 60 | 60 H. influenzae52 solitarily3 + C. albicans3 + T. vaginalis1 + N. gonorrhoeae1 + C. trachomatis | NP/37M, 20F and 3 NP | NP | NP | 37 Urethral exudate19 Vaginal and/or cervical exudate3 urine1 Bartholin's gland | Culture and biochemical tests | NP |
| Andreu A (1989)35 | 10 | 7 H. parainfluenzae3 H. influenzae7 solitarily3 + other* | NP/M | NP | 7 urethritis | Urethral exudate | NP | NP |
| 20 | 15 H. influenzae5 H. parainfluenzae + others* | NP/F | 5 IUD carriers | 8 vaginitis, 3 salpingitis and 9 NP | Vaginal or endocervical exudate | NP | NP | |
| Drouet EB (1989)36 | 244 | 216 H. parainfluenzae28 H. influenzae | NP | NP | NP | NP | NP | NP |
| Houang E (1989)37 | 73 | H. parainfluenzae | NP/71M and 2 F | NP | NP | 71 urethral exudate2 vaginal exudate | MicroScan | NP |
| Martel AY (1989)38 | 103 | Haemophilus spp. | NP/56M, 33F and 14 NP | NP | NP | 55 genital exudate48 rectal exudate | Biochemical tests | NP |
| Leiberman JR (1989)39 | 1 | H. influenzae | 35/F | 16 weeks pregnant. | Amnionitis | Amniotic fluid | Culture | NP |
| van Bosterhaut B (1990)40 | 3 | H. influenzae | NP/F | NP | 3 bartholinitis | Gland exudate | Culture | NP |
| Christensen JJ (1990)41 | 17 | Haemophilus spp. | NP/F | NP | Bartholinitis, salpingitis and/or vaginal discharge | NP | NP | NP |
| Quentin R (1990)42 | 8 | H. influenzae | NP/F | Bartholin's gland cyst or abscess surgery | Bartholinitis | Aspirated cyst or abscess | NP | NP |
| Silverberg K (1990)43 | 1 | H. influenzae | 17/F | 29 weeks pregnant. | Chorioamnionitis | Amniotic fluid and placenta | Culture | Ampicillin, gentamicin, clindamycin |
| Pinhas-Hamiel O (1991)44 | 1 | H. influenzae | 28/F | 13 weeks pregnantPrevious risky sexual relationship | Septic abortion | Blood cultures | Culture | NP |
| Facinelli B (1991)45 | 1 | H. parainfluenzae | NP | Risky sexual relationship | Urethritis | NP | NP | NP |
| Bendig JW (1991)46 | 2 | H. influenzae | 35/F, 29/F | 1 IUD | 2 purulent salpingitis | Peritoneal and tubal exudate | Culture | Drainage, ceftriaxone, metronidazole |
| Lefevre JC (1991)47 | 21 | H. parainfluenzae | NP/M | NP | Urethritis | Urethral exudate | NP | NP |
| Bosch J (1991)48 | 9 | H. influenzae | NP/F | NP | 7 Bartholin's gland abscesses1 post-caesarean section endometritis1 chorioamnionitis | Vaginal and gland exudate | NP | Ampicillin |
| Rusin P (1991)49 | 13 | H. influenzae | NP/F | NP | 13 endometritis/chorioamnionitis | NP | NP | NP |
| Mazor M (1991)50 | 1 | H. influenzae | 28/F | 29 weeks pregnant.Premature rupture of membranes | Amnionitis | Amniotic fluid and neonatal blood | Culture | Ampicillin and gentamicin |
| Kragsberg P (1993)51 | 6 | H. influenzae | NP/F | 6 pregnancies1 Recurrent endometritis | 3 tubo-ovarian abscesses2 septic abortions1 postpartum sepsis | Abscess exudateVaginal exudate | Culture | Cephalosporin, ampicillin, metronidazole, doxycycline |
| Ault KA (1993)52 | 3 | H. influenzae | 18F, 22F, 29F | 3 Pregnancies (29, 31 and 31 weeks) | Amnionitis | Placenta and maternal blood | Culture | Vancomycin and gentamicinAmpicillin and gentamicinCeftizoxime |
| Kinney JS (1993)53 | 6 | H. influenzae | NP/F | Pregnant 24–40 weeks | Chorioamnionitis | Placenta and neonatal blood | Culture | 1 Ampicillin, 1 cephalosporin |
| Gill MV (1995)54 | 1 | H. influenzae | 36/F | 35 weeks pregnant, placenta percreta | Chorioamnionitis | Blood cultures and cervical exudate | Culture | Ceftazidime and aztreonam |
| Llanes Caballero R (1996)55 | 1 | H. influenzae | NP/M | Previous schistosomiasis | Discomfort while urinating and purulent urethral discharge | Urethral exudate | NP | Norfloxacin |
| Pena MJ (1996)56 | 20 | H. influenzae | NP/F | Prepubescent girls | Vulvovaginitis | Vaginal exudate | NP | NP |
| Vázquez F (1996)57 | 155 | 100 H. parainfluenzae45 H. influenzae10 Haemophilus spp.21 solitarily134 + others* | 2 M, 11F and 142 NP | Female sex workers and men with urethritis | 8 urethritis2 epididymo-orchitis9 cervicitis and/or vaginitis2 Bartholin's gland abscesses134 NP | NP | Culture | NP |
| Carmeci C (1997)58 | 1 | H. influenzae | 48/F | NP | Acute salpingitis and septic shock | Peritoneal exudate and blood cultures | Culture | Salpingo-oophorectomy and ceftriaxone |
| Cox RA (1997)4 | 11 | H. influenzae | <14/ F | NP | Vulvovaginitis | Vaginal and vulvar exudate | Culture | Amoxicillin |
| García E (1997)59 | 30 | 18 H. influenzae | NP/F | Pregnant, parturient, puerperal | 1 abortion2 antepartum deaths1 postpartum death14 live newborns (5 confirmed infection and 5 suspected infection and 4 healthy) | Vaginal exudate taken intrapartum or in the immediate postpartum | NP | NP |
| 12 H. parainfluenzae | 1 antepartum death12 live newborns (10 suspected infection) | |||||||
| Aydin MD (1998)60 | 19 | 11 H. parainfluenzae8 H. influenzae | NP/M | NP | Urethritis | Urethral exudate | Biochemical tests | NP |
| Rodríguez-Guardado (2000)61 | 1 | H. influenzae | 36/F | NP | Bilateral salpingitis | Peritoneal exudate | Culture | Salpingectomy, clindamycin, gentamicin |
| Cherpes TL (2002)62 | 1 | H. influenzae | 39/F | 8 weeks pregnant | Septic abortion. Acute chorioamnionitisLow-grade fever, vaginal bleeding, and headache | Blood culturePlacental biopsy | NP | Ampicillin+gentamicin+clindamycinCeftriaxoneLevofloxacin |
| Cox RA (2002)63 | 39 | 38 H. influenzae1 H. parainfluenzae8 + mixed anaerobic flora | 18 months -11 years/ F | 14 recurrent vulvovaginitis5 two previous episodes of vulvovaginitis due to H. influenzae, 1 three previous episodes | Vulvovaginitis: vaginal discharge and irritation | Vaginal and vulvar exudate | Culture | 19 amoxicillin9 amoxicillin/clavulanic acid6 trimethoprim6 miconazole2 metronidazole2 clarithromycin, erythromycin |
| Campos J (2003)64 | 2 | H. influenzae serotype e | NP/F | NP | Vaginitis | NP | NP | NP |
| Cuadros J (2004)65 | 12 | H. influenzae | 2−12/ F | NP | Vulvovaginitis | Vaginal exudate | NP | NP |
| Mikamo H (2005)66 | 1 | H. influenzae | NP/F | NP | Bartholinitis | Gland exudate | Culture | Cefteram pivoxil |
| Tanaka K (2005)67 | 8 | H. influenzae | NP/F | NP | Bartholin's gland abscess | Gland exudate | Culture and PCR | NP |
| Iser P (2005)68 | 2 | H. influenzae | NP/M | Heterosexual | Nongonococcal urethritis | Urethral exudate | Culture | NP |
| Tsai HY (2006)69 | 1 | H. influenzae | NP/F | Prepubescent girl. No sexual relations, no abuse. | Vulvovaginitis, profuse vaginal discharge | Vaginal exudate | NP | NP |
| Varela JA (2006)70 | 12 | Haemophilus spp. | NP/M | HeterosexualCouple with cervical intraepithelial neoplasia (CIN) | Asymptomatic | Urethral exudate | Culture | NP |
| Kohlberger P (2007)71 | 11 | H. influenzae | NP/F | Girls with a history of sexual abuse | NP | Vaginal exudate | NP | NP |
| Santiago JL (2008)72 | 1 | H. parainfluenzae | 41/M | HeterosexualUnprotected oral sex with a sex worker | Dysuria, urethral discharge and inguinal adenopathy.Conjunctival hyperemia with purulent exudate, tarsal follicular reaction, blepharitis and palpebral oedema | Urethral and conjunctival exudate | Culture and biochemical tests | Ceftriaxone+doxycycline |
| Orellana MA (2009)73 | 36 | 22 H. parainfluenzae14 H. influenzae | NP/M | NP | NP | Urethral exudate | API NH® test | NP |
| Sikanić-Dugić N (2009)74 | 5 | H. influenzae | 2−8/ F | NP | Vulvovaginitis | Vaginal exudate | Culture | NP |
| Kakisi (2010)75 | 1 | H. influenzae | 42F | NP | Tubo-ovarian abscess | Abscess exudate | Culture | Surgery and cefoxitin/metronidazole |
| Glover WA (2011)8 | 1 | H. quentini | 28/M | Previous episodes of urethritis and prostatitis.Unprotected sex | Scarce urethral discharge, painful ejaculation | Urethral exudate | 16S rRNA sequencing | Ceftriaxone+azithromycin |
| 1 | H. quentini | 30/M | NP | NP | NP | |||
| 1 | H. quentini +N. gonorrhoeae | 32/M | NP | NP | NP | |||
| Mc Kechnie ML (2011)76 | 11 | H. influenzae | 2<25,5 25−29,4>35/ F | NP | 7 symptomatic4 asymptomatic | Vaginal exudate Endocervical exudateUrine | PCR | NP |
| Orellana MA (2011)77 | 77 | 45 H. parainfluenzae32 H. influenzae4 + C. trachomatis2 + U. urealyticum | >15/M | NP | Urethritis | Urethral exudate | API NH® test | NP |
| Calner PA (2012)78 | 1 | H. influenzae | 36/F | 16 weeks pregnant | Septic abortion | Blood cultures | Culture | Curettage, ampicillin, gentamicin, clindamycin |
| Ranđelović G (2012)79 | 2 | H. influenzae | 2−12/ F | NP | Vulvovaginitis | Vaginal exudate | NP | NP |
| Martin D (2013)80 | 1 | H. influenzae | 48/ F | Uterine leiomyoma. No IUD | Acute endometritisLower abdominal pain and fever | Endometrial biopsy | PCR | NP |
| Tinguely R (2013)81 | 1 | H. parainfluenzae +N. gonorrhoeae | NP/M | Homosexual.Recently treated syphilis | Urethritis | Urethral exudate | 16S rRNA sequencing | Ciprofloxacin |
| 1 | Homosexual | PCR | Ciprofloxacin+doxycycline (NR)Ceftriaxone+azithromycin | |||||
| Mendz GL (2014)82 | 1 | H. parainfluenzae +C. curvus | 29/ F | Pregnant (25 weeks) | Chorioamnionitis, premature rupture of membranes, neonatal sepsis | Vaginal exudate | 16S rRNA sequencing | Penicillin+gentamicin+metronidazole |
| Cardines R (2015)83 | 46 | 37 H. parainfluenzae7 H. pittmaniae2 H. parahaemolyticus | 32.5/ F | Pregnant (25−38 weeks) | Asymptomatic | Vaginal exudate | 16S rRNA sequencing | NP |
| Hsu MS (2015)84 | 1 | H. parainfluenzae + Enterococcus spp. | 29/M | Homosexual, unprotected sex | Dysuria, purulent urethral discharge | Urethral exudate | 16S rRNA sequencing | Ceftriaxone+doxycycline |
| 1 | H. parainfluenzae +N. gonorrhoeae +M. morganii | 32/M | Dysuria, frequency, purulent urethral discharge | Ceftriaxone+doxycycline | ||||
| 1 | H. parainfluenzae + Enterococcus spp. | 27/M | Dysuria, purulent urethral discharge, inguinal adenopathy | Clarithromycin | ||||
| Deza G (2016)85 | 52 | 45 H. parainfluenzae7 H. influenzae24 + other microbes10 + N. gonorrhoeae8 + C. trachomatis3 + C. albicans2 + G. vaginalis1 + U. urealyticum | 31.8/M | 31 (60%) homosexual and 21 (40%) heterosexual4 (8%) HIV9 (17%) previous STI52 (100%) unprotected oral sex29 (55%) anal sex, 44% unprotected19 (36%) vaginal sex, 31% unprotected | 37 (71%) purulent urethral discharge13 (25%) non-purulent urethral discharge2 (4%) dysuria without urethral discharge | Urethral exudate | API20E | 48 (92%) ceftriaxone+azithromycin/doxycycline2 (4%) doxycycline1 (2%) azithromycin1 (2%) ciprofloxacin |
| 28 solitarily23 H. parainfluenzae5 H. influenzae | 33.7/M | 15 (54%) homosexual and 13 (46%) heterosexual.3 (11%) HIV5 (18%) previous STI28 (100%) unprotected oral sex (5 (18%) only sexual practice)13 (46%) anal sex, 39% unprotected11 (39%) vaginal sex, 32% unprotected | 18 (64%) purulent urethral discharge9 (32%) non-purulent urethral discharge1 (4%) dysuria without urethral discharge | 28 (100%) ceftriaxone+azithromycin/ doxycycline | ||||
| Frølund M (2016)86 | 9 | 6 H. influenzae solitarily2 + adenovirus1 + M. genitalium | NP/M | NP | Acute urethritisDysuria and urethral discharge | First morning urine | PCR | NP |
| Ito S (2016)87 | 22 | 21 H. influenzae solitarily1 + U. urealyticum | 7<31 and14>31/M | 11 married and 10 single.12 prior urethritis.21 unprotected oral sex(12 exclusively).9 unprotected oral and vaginal sex.13 sex worker partner | 21 urethral discharge (13 moderate and 8 scant) in 10 serous,6 mucoid and 5 mucopurulent15 urethral irritation12 moderate dysuria3 meatitis and/or balanitis3 pharyngeal discomfortIncubation < 7 days | Urethral exudate | NP | NP |
| Kim H (2016)88 | 1 | H. influenzae | 0−9/ F | Prepubescent girl | Vulvovaginitis | Vaginal or vulvar exudate | Culture | NP |
| You C (2016)89 | 8 | H. influenzae | NP/M | NP | Urethritis | First morning urine | 16S RNA sequencing | NP |
| Porter M (2016)90 | 31 | H. influenzae | 21−29/F | 31 pregnancies | 31 Chorioamnionitis | Placenta | Culture | NP |
| Baba H (2017)91 | 1 | H. influenzae | 31/ F | 17 weeks pregnant.History of upper respiratory infection with cough. | Septic abortion.High fever, vaginal bleeding, and abdominal pain | Vaginal exudateBlood culture | Culture, MALDI-TOF, PCR. | Piperacillin/tazobactamCefotaxime+metronidazole |
| Chen X (2017)92 | 1 | H. influenzae | 6/ F | History of a foreign body in the vagina.Recurrent and chronic rhinitis. In nasal exudate, H. influenzae is isolated, derived from the same clone as the one detected in the vagina | Vulvovaginitis associated with rhinitis | Vaginal exudate | NP | NP |
| Deguchi T (2017)93 | 73 | H. influenzae | NP/M | NP | 67 acute urethritis4 acute epididymitis2 urethritis and epididymitis | Urethral exudate | NP | NP |
| Ito S (2017)1 | 68 | 54 H. influenzae solitarily5 + U. urealyticum4 + U. urealyticum + M. hominis2 + U. parvum1 + C. trachomatis1 + N. gonorrhoeae | 35/M | Urethritis | Urethral exudate | HN-20 Rapid system identification test | 43 azithromycin (5 NR)20 sitafloxacin3 levofloxacin2 ceftriaxone | |
| 6 | H. influenzae | NP/M | NP | Epididymitis | NP | |||
| 1 | Acute prostatitis | |||||||
| Li JP (2017)94 | 110 | H. influenzae | NP/F | Prepubescent girls | Vulvovaginitis | Vaginal exudate | Culture | 55 ofloxacin49 β-lactams6 NP |
| Caballero Mateos AM (2018)95 | 1 | H. parainfluenzae + C. trachomatis L2 (lymphogranuloma venereum) | 34/M | Homosexual, unprotected sexHepatitis B previously treated. Secondary syphilis.Drug addict. | Proctalgia, constipation, rectal bleeding, fever.Rectal ulcer | Rectal biopsy | NP | Doxycycline |
| Horie K (2018)96 | 1 | H. quentini + Streptococcus spp. +M. hominis +U. urealyticum | 34/M | Heterosexual. SinglePrevious urethritis (no other previous STIs)Casual partners | Moderate mucoid urethral dischargeIncubation 12 days | Urethral exudate | 16S rRNA sequencing | Azithromycin |
| 1 | H. quentini | 24/M | Heterosexual SingleNo previous STIsHIV negativeUnprotected oral sex with a sex worker | Scant mucoid urethral discharge, dysuriaIncubation 4 days | Sitafloxacin | |||
| 1 | H. quentini + Streptococcus spp. + | 30/M | Heterosexual SingleNo previous STIsHIV negativeUnprotected oral and vaginal sex with a stable partner and other partners | Condyloma acuminatum on the glansScant serous urethral discharge, urethral irritation, dysuria | Levofloxacin | |||
| Kondo H (2018)97 | 1 | H. influenzae + Streptococcus spp. +U. urealyticum | 32/M | Heterosexual Married4 previous episodes of urethritis (no other STIs)Multiple sexual partners | Urethral irritation, mucopurulent urethral discharge | Urethral exudate | AzithromycinND | |
| 1 | H. influenzae | 27/M | Heterosexual MarriedNo previous STIsStable sexual partner | Urethral irritation, scant mucoid urethral dischargeIncubation 16 days | ||||
| 1 | H. influenzae +S. agalactiae | 30/M | Heterosexual SingleNo previous STIsUnprotected oral sex with a sex worker | Urethral irritation, scant mucoid urethral dischargeIncubation 5 days | ||||
| 1 | H. influenzae | 29/M | Heterosexual Married1 previous episode of urethritis (no other STIs)Unprotected oral sex with a sex worker | Dysuria, scanty mucoid urethral dischargeIncubation 12 days | ||||
| 1 | H. influenzae | 21/M | Heterosexual Married1 previous episode of urethritis (no other STIs)Unprotected oral and vaginal sex with a sex worker | Urethral irritation, mucopurulent urethral dischargeIncubation 5 days | ||||
| 1 | H. influenzae | 25/M | Heterosexual MarriedNo previous STIsUnprotected oral sex with a sex worker | Dysuria, urethral irritation, mucopurulent urethral dischargeIncubation 9 days | ||||
| Saxena A (2018)98 | 1 | H. parainfluenzae | 33/M | No previous STIsHIV negativeUnprotected oral sex with a sex worker | Urethral pruritus, urethral discharge | Urethral exudate | MALDI-TOF | Norfloxacin+amoxic-clav+fluconazole (NR)Azithromycin+ciprofloxacin |
| Cevik M (2019)99 | 1 | H. influenzae | 35/ F | 14 weeks pregnant | Septic abortion. Acute chorioamnionitisAbdominal pain, vaginal discharge/bleeding, fever. | Placental biopsyBlood cultureCervical exudate | NP | β-lactams |
| 1 | 22/ F | 14 weeks pregnant | ||||||
| 1 | 33/ F | 20 weeks pregnant.Premature rupture of membranesPrevious abortion. History of chorioamnionitis | ||||||
| Magdaleno Tapial J (2019)100 | 38 | 32 H. parainfluenzae5 H. influenzae1 H. haemolyticus15 Haemophilus spp. solitarily9 + M. hominis/ Ureaplasma spp7 + Chlamydia6 + Neisseria2 + M. genitalium | 30.5/35 (92%) M3 (8%) F | 21 (55%) M homosexual13 (34%) M heterosexual3 (8%) F heterosexual1 (3%) NP5 (13%) HIV20 (57%) previous STI38 (100%) unprotected oral sex | 22 (58%) purulent urethral discharge6 (16%) dysuria10 (26%) asymptomatic, risky sexual contact | Urethral exudate | NP | 17 (45%) ceftriaxone+azithromycin12 (31%) ceftriaxone+doxycycline5 azithromycin4 doxycycline6NR |
| Wang HJ (2019)101 | 230 | H. influenzae | NP/F | Prepubescent girls | Vulvovaginitis | Vaginal exudate | Vitek culturesystem NH | NP |
| Alsuhaibani MA (2019)102 | 1 | H. parainfluenzae | 26/F | Pregnant | Chorioamnionitis | Placenta and neonatal blood | Culture | Cefotaxime+gentamicin |
| Ducours M (2020)103 | 1 | H. parainfluenzae +S. hominis+E. faecalis+S. anginosus+p. harei | 20/M | HeterosexualUnprotected vaginal sex | Urethral discharge | Urethral exudate | MALDI-TOF | Ceftriaxone+azithromycin |
| 1 | H. parainfluenzae +N. gonorrhoeae +S. epidermidis +S. anginosus+S. mitis | 31/M | HomosexualUnprotected anal sex | Dysuria, urethral discharge | Ceftriaxone+azithromycin | |||
| 1 | H. parainfluenzae +M. genitalium +E. faecalis+S. mitis | 37/M | Heterosexual | Dysuria, urethral discharge | Minocycline+pristinamycin | |||
| 1 | H. parainfluenzae +N. gonorrhoeae +S. haemolyticus+Corynebacterium sp. | 43/M | HomosexualHIVUnprotected oral and anal sex | Dysuria, urethral discharge | Ceftriaxone+doxycycline | |||
| 1 | H. parainfluenzae +S. haemolyticus +E. faecalis | 30/M | Homosexual. PrEPUnprotected oral and anal sex | Dysuria, urethral discharge | Cotrimoxazole (NR)Gentamicin +ciprofloxacin | |||
| 1 | H. parainfluenzae +S. haemolyticus+S. mitis | 28/M | HomosexualUnprotected anal sex | Dysuria, urethral discharge | Ceftriaxone+doxycycline | |||
| 1 | H. parainfluenzae +S. haemolyticus | 27/M | NP | NP | NP | |||
| 1 | H. influenzae | 55/M | Homosexual. PrEPUnprotected oral and anal sex | Urethral discharge | Ceftriaxone+azithromycin | |||
| 1 | H. parainfluenzae +N. gonorrhoeae+E. coli | 33/M | Homosexual. PrEPUnprotected oral and anal sex | Dysuria, urethral discharge | Ceftriaxone | |||
| Nishimura Y (2020)104 | 1 | H. influenzae | 51/ F | Polypoid adenomyomaEarly stage endometrioid adenocarcinoma | Microabscesses in adenomyoma. | Vaginal exudateBlood culture | MALDI-TOF | Ceftriaxone+hysterectomy |
| Sierra Y (2020)105 | 175 | H. parainfluenzae ± NP125 NOT multiresistant50 multiresistant30 H. parainfluenzae +C. trachomatis/N. gonorrhoeae/T. pallidum10 NP10 H. parainfluenzae solitarily | 37.8/97 (55%) M78 (45%) FNPNPNPNP42/M | NPNPNPNPNP4 homosexuals, 4 heterosexuals and 2 NP.4 HIV6 previous STI8 unprotected sex with a stranger, 1 unprotected sex with a stable partner and 1 NP | NPNP26 urethritis and 24 NP16 urethritis and 14 NP5 asymptomatic and 5 NP10 (100%) urethritis | 79 (45%) urethral exudate62 (35%) vaginal13 (7%) preputial9 (5%) cervical6 (3%) rectal3 (2%) genital ulcer1 (1%) pharyngeal1(1%) semen1 (1%) urineNPNPNPNP10 urethral exudate | MALDI-TOF | NPNPNPNPNP5 ceftriaxone+azithromycin2 ceftriaxone+doxycycline1 ceftriaxone+azithromycin+doxycycline1 amox-clav |
| Snirivasan S (2020)3 | 40 | H. influenzae | NP/M | 23 heterosexual17 homosexual | 16 urethritis and 7 asymptomatic16 urethritis and 1 asymptomatic | First morning urine | 16S rRNA sequencing | NP |
| Vives A (2020)106 | 30 | 19 H. parainfluenzae10 H. influenzae1 Haemophilus sp.25 solitarily5 + other microbes4 + C. trachomatis1 + N. gonorrhoeae | 36.6/M | 17 (57%) heterosexual, 8 (27%) homosexual, 2 (7%) bisexual and 3 (10%) NP3 (10%) HIV with no detectable viral load13 (43%) previous STI21 (75%) oral sex, 15 (54%) vaginal sex, and 10 (36%) anal sex.N.o sexual partners 3.5 [1–20] | 13 (43%) urethral discharge7 (23%) dysuria5 (17%) testicular pain2 (7%) genital ulcer1 (4%) hematospermia1 (4%) painful ejaculation1 (4%) lower abdominal pain | Urethral exudate | API NH® test | Ceftriaxone+azithromycin/doxycyclineAzithromycin±ciprofloxacinCiprofloxacinDoxycycline8 NR |
| Hu BF (2021)107 | 140 | H. influenzae | 5.8/F | NP | Symptomatic vulvovaginitis | Vaginal exudate | Vitek | Ampicillin |
| Bruins MJ (2021)108 | 127 | H. influenzae | 37 (<12 y)/F2(12−17 y)/F45(18−51 y)/F43(>51)/F | NP | Symptomatic vulvovaginitis | Vaginal exudate | Culture | NP |
F: woman; M: male; NP: not provided; NR: no response to treatment.
Most of the studies adequately described the method used in the laboratory for the isolation of Haemophilus. Until 2011, mainly culture techniques and biochemical tests were used. From 2011 onwards, molecular biology techniques, such as PCR or the sequencing of the 16S subunit of rRNA, became more prominent. More recently, mass spectrophotometry has been used (MALDI -TOF).
Regarding patient gender, 34.8% (835/2397) were men; 48.3% were women (1158/2397) and 16.9% (404/2397) were not reported. Only half of the studies provided data on patient age. The men were all adults with a mean age of 30 years20–55. The women were divided into two groups, one of children under 14 years of age (53.9%; 624/1158) and another of adults (14 years of age or older, 28.4%; 329/1158). The age was not recorded in 17.7% of women (205/1158).
In the group of men, H. parainfluenzae (44.1%; 367/835) and H. influenzae (42.6%; 356/835) were detected with almost equal frequency. The vast majority were diagnosed with urethritis. The most frequent clinical manifestations were urethral discharge, purulent or not, dysuria and urethral pruritus or irritation. Regarding sample type, in the vast majority of cases it was urethral exudates. The most frequently administered treatment was ceftriaxone with azithromycin and/or doxycycline, although the treatment was only specified in 40% (15/37) of the publications referring to men.
We subdivided the group of adult women according to whether the infection occurred during pregnancy (52.9%; 174/329) or not (47.1%; 155/329). In the latter, there were episodes of vaginitis, bartholinitis, salpingitis, endometritis or tubal abscesses. The absence of a significant portion of data prevents us from drawing conclusions about the prevalence of a specific species of Haemophilus. In pregnant women, almost all the studies refer to septic abortion, acute chorioamnionitis, premature rupture of membranes and neonatal sepsis. H. influenzae was detected more frequently (64.9%; 113/174) than H. parainfluenzae (30.5%; 53/174). H. influenzae was isolated in all the samples from girls (vaginal or vulvar exudates). Most of them presented vaginal discharge, vulvar irritation or pruritus and did not have predisposing factors. Antibiotic treatment included β-lactams or quinolones.
Samples of rectal origin only represented 2.3% (55/2397). The clinical and epidemiological information available on these patients was very scant, with the exception of one reported case7.
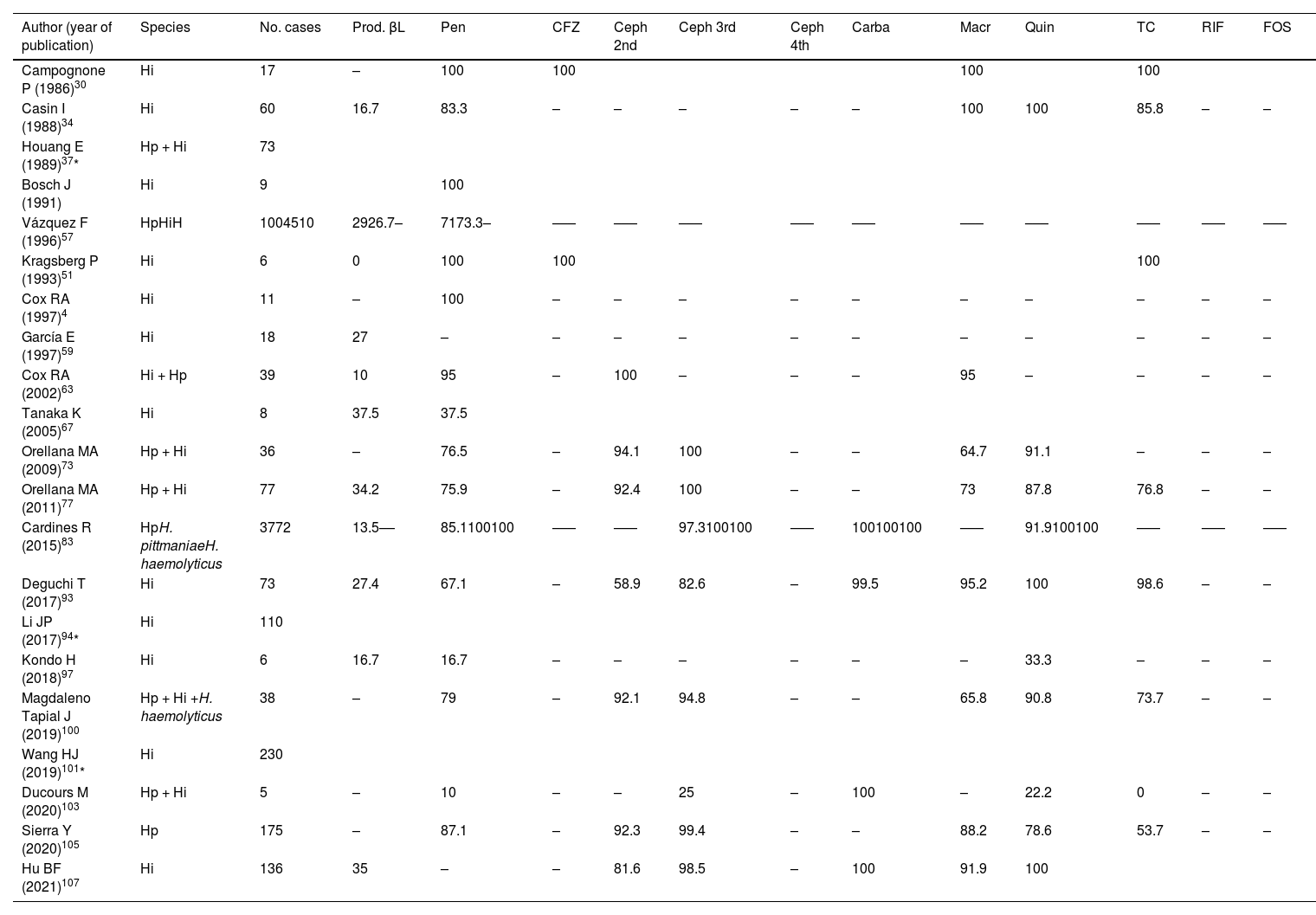
Table 2 summarises the information on the susceptibility ofHaemophilus to different groups of antibiotics (penicillin, carbapenems, tetracycline, quinolones, first-, second- and third-generation cephalosporins) contributed by 21 of the 67 studies. Four of these were excluded because they did not disaggregate the results by sample origin, as were those with fewer than five reported cases to avoid bias. Overall susceptibility of Haemophilus spp. to penicillins was 78.9%, and the overall rate of β-lactamase production was 26.4%. It should be remembered that although the production of β-lactamases is the main mechanism of resistance in this genus, there are also strains that do not produce β-lactamases but have alterations in the penicillin-binding proteins with a low affinity for β-lactams and even a combination of both mechanisms109.
Antibiotic susceptibility (%) of Haemophilus spp. isolates.
| Author (year of publication) | Species | No. cases | Prod. βL | Pen | CFZ | Ceph 2nd | Ceph 3rd | Ceph 4th | Carba | Macr | Quin | TC | RIF | FOS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Campognone P (1986)30 | Hi | 17 | – | 100 | 100 | 100 | 100 | |||||||
| Casin I (1988)34 | Hi | 60 | 16.7 | 83.3 | – | – | – | – | – | 100 | 100 | 85.8 | – | – |
| Houang E (1989)37* | Hp + Hi | 73 | ||||||||||||
| Bosch J (1991) | Hi | 9 | 100 | |||||||||||
| Vázquez F (1996)57 | HpHiH | 1004510 | 2926.7– | 7173.3– | ––– | ––– | ––– | ––– | ––– | ––– | ––– | ––– | ––– | ––– |
| Kragsberg P (1993)51 | Hi | 6 | 0 | 100 | 100 | 100 | ||||||||
| Cox RA (1997)4 | Hi | 11 | – | 100 | – | – | – | – | – | – | – | – | – | – |
| García E (1997)59 | Hi | 18 | 27 | – | – | – | – | – | – | – | – | – | – | – |
| Cox RA (2002)63 | Hi + Hp | 39 | 10 | 95 | – | 100 | – | – | – | 95 | – | – | – | – |
| Tanaka K (2005)67 | Hi | 8 | 37.5 | 37.5 | ||||||||||
| Orellana MA (2009)73 | Hp + Hi | 36 | – | 76.5 | – | 94.1 | 100 | – | – | 64.7 | 91.1 | – | – | – |
| Orellana MA (2011)77 | Hp + Hi | 77 | 34.2 | 75.9 | – | 92.4 | 100 | – | – | 73 | 87.8 | 76.8 | – | – |
| Cardines R (2015)83 | HpH. pittmaniaeH. haemolyticus | 3772 | 13.5–– | 85.1100100 | ––– | ––– | 97.3100100 | ––– | 100100100 | ––– | 91.9100100 | ––– | ––– | ––– |
| Deguchi T (2017)93 | Hi | 73 | 27.4 | 67.1 | – | 58.9 | 82.6 | – | 99.5 | 95.2 | 100 | 98.6 | – | – |
| Li JP (2017)94* | Hi | 110 | ||||||||||||
| Kondo H (2018)97 | Hi | 6 | 16.7 | 16.7 | – | – | – | – | – | – | 33.3 | – | – | – |
| Magdaleno Tapial J (2019)100 | Hp + Hi +H. haemolyticus | 38 | – | 79 | – | 92.1 | 94.8 | – | – | 65.8 | 90.8 | 73.7 | – | – |
| Wang HJ (2019)101* | Hi | 230 | ||||||||||||
| Ducours M (2020)103 | Hp + Hi | 5 | – | 10 | – | – | 25 | – | 100 | – | 22.2 | 0 | – | – |
| Sierra Y (2020)105 | Hp | 175 | – | 87.1 | – | 92.3 | 99.4 | – | – | 88.2 | 78.6 | 53.7 | – | – |
| Hu BF (2021)107 | Hi | 136 | 35 | – | – | 81.6 | 98.5 | – | 100 | 91.9 | 100 |
Amin: aminoglycosides; ATM: aztreonam (monobactam); Carba: carbapenems; Ceph 2nd: 2nd-generation cephalosporins; Ceph 3rd: 3rd-generation cephalosporins; Ceph 4th: 4th-generation cephalosporins; CFZ: cefazolin (1st-generation cefalosporin); CHL: chloramphenicol; FOS: fosfomycin; Hi: Haemophilus influenzae; Hp: Haemophilus parainfluenzae; Macr: macrolides; Pen: penicillins; Prod. βL: β-lactamase production; Quin: quinolones; RIF: rifampicin; SXT: trimethoprim-sulfamethoxazole; TC: tetracyclines.
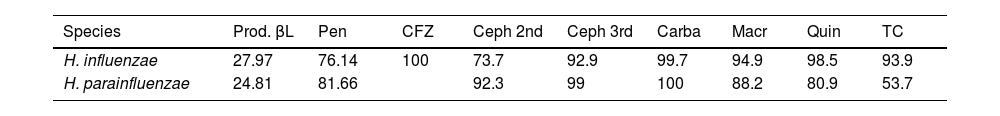
In addition, susceptibility to these antibiotics was determined separately for each species. These data are shown in Table 3.
Rates of antibiotic susceptibility and presence of β-lactamases separated by species (%).
| Species | Prod. βL | Pen | CFZ | Ceph 2nd | Ceph 3rd | Carba | Macr | Quin | TC |
|---|---|---|---|---|---|---|---|---|---|
| H. influenzae | 27.97 | 76.14 | 100 | 73.7 | 92.9 | 99.7 | 94.9 | 98.5 | 93.9 |
| H. parainfluenzae | 24.81 | 81.66 | 92.3 | 99 | 100 | 88.2 | 80.9 | 53.7 |
Carba: carbapenems; Ceph 2nd: 2nd-generation cephalosporins; Ceph 3rd: 3rd-generation cephalosporins; CFZ: cefazolin (1st-generation cephalosporin); Macr: macrolides; Pen: penicillins; Prod. βL: β-lactamase production; Quin: quinolones; TC: tetracyclines.
It is difficult to demonstrate the pathogenic role of microorganisms that can be colonisers and present together with other species, as in the case of HSNOTD and in particular Haemophilus influenzae. There are few studies with a control group on the prevalence of the latter in men with urethritis, although in one of them3, an association is demonstrated with non-gonococcal urethritis in both MSM and heterosexuals, which would support its aetiological and pathogenic role in urethritis. Doubts remain as to the pathogenic role of the rest of the HSNOTD species since there are no rigorous studies with valid methodology, and most of them are case series.
The acquisition mechanism varies depending on the characteristics of the patient. For example, in girls with no history of sexual intercourse or abuse, there could be an auto-inoculation mechanism from the nasal location to the vaginal area. In contrast, in adults, the transmission route is predominantly sexual. One of the most relevant data items we can extract from this bibliographical review is the high frequency with which urethritis caused by HSNOTD is associated with the practice of unprotected oral sex, which in many cases is the only form of exposure reported. Genital infection by HSNOTD seems to be associated with sexual contact with multiple sexual partners and with the domain of prostitution. However, it is important to be cautious when drawing conclusions about possible predisposing factors to genital infection by HSNOTD, since the information provided by the different publications is scant. Regarding the sexual orientation of male patients, 53.3% were heterosexual and 46.7% homosexual, but the information available is very limited and does not allow us to conclude that the risk of infection is related to sexual orientation. The same occurs with previous having had sexually transmitted infections (urethritis, HIV, syphilis, etc.) as a risk factor. We found the same number of patients with a history of STIs as without them, although once again we cannot draw definitive conclusions due to the general lack of information. The low number of publications in which HSNOTD is considered a pathogenic agent causing proctitis is striking.
The susceptibility rates of Haemophilus against cephalosporins, especially third-generation, are very high, which together with amoxicillin/clavulanic acid are the treatments of choice. Taking into account the antibiotic susceptibility profile of all the species described above, we can deduce that the empiric treatment administered for STIs could be more effective against H. influenzae than against H. parainfluenzae, since the latter presents lower rates of susceptibility to macrolides, tetracyclines and quinolones.
Traditionally, vulvovaginitis in girls has been treated with penicillins, and a great tendency to a recurrence of these episodes has been described3, which could be due to the ineffectiveness of empiric treatment, since the susceptibility rate of H. influenzae to these antibiotics is only 72.3%. This hypothesis is reinforced by the fact that the recurrence rate is higher in girls who had received penicillins in the preceding months to treat infections in other locations. One alternative to penicillins are cephalosporins, which show good susceptibility rates and do not have the possible side effects of other antibiotics, such as quinolones, in children. Finally, one of the works reviewed46 demonstrates the frequency with which Haemophilus spp. is detected in the vaginal exudate of asymptomatic pregnant women, with percentages close to 10%. This could entail a greater risk of vertical transmission and infectious complications both at maternal level and regarding neonatal sepsis, and prophylactic eradication may be considered as a strategy. However, a deeper analysis of the risk-benefit ratio of this intervention, which could increase antibiotic pressure, is necessary.
This review has several limitations. An extensive search was carried out. However, articles in Spanish and English were included and we did not have access to a series of published studies, listed at the beginning of the article, meaning that not all published studies may not have been included. In addition, the wide variability of data published between articles regarding epidemiological, microbiological and antibiotic susceptibility data makes it difficult to analyse the data homogeneously and therefore draw conclusions. Furthermore, the methodology of most of the included studies is outmoded and the aetiological role of these microorganisms cannot therefore be firmly established.
Following the review, we can highlight, in HSNOTD, the role ofHaemophilus influenzae as an aetiological agent in cases of non-gonococcal urethritis in men, both MSM and heterosexuals, and therefore the usefulness of systematically searching for this microorganism in this entity, as it can explain a significant percentage of urethritis without microbiological isolation. It proved impossible to find consistently described epidemiological risk factors and/or an acquisition mechanism, hence more studies are needed. On the other hand, taking the antibiotic resistance profile into account, third-generation cephalosporins, amoxicillin-clavulanic acid and quinolones are postulated as the most successful eradication options. With regard to azithromycin and doxycycline, which are widely used in the empiric treatment of STIs, we did not find significant resistance rates in the review, although we are seeing a growing trend of strains with a higher resistance profile and higher minimum inhibitory concentrations for doxycycline110.
Conflicts of interestThe authors declare that they have no conflicts of interest.