To evaluate the beneficial effects of exogenous NO and an inhibitor of the COX2, and their action levels in a model of SIRS/bacterial translocation (BT) induced by Zymosan A®.
Material and methodsNinety Wistar rats were submitted to different treatments, and after 12h and 24h they were anaesthetized in order to collect blood, mesenteric lymph nodes, and kidney for subsequent biochemical analyses and microbiological examinations.
TreatmentsA nitric oxide donor, Molsidomine®, was compared with a COX2 inhibitor, Celecoxib®.
MethodsZymosan A® was administered to Wistar rats. The animals were divided into 6 groups: one group for survival study, Group (1) No manipulation (BASAL); Group (2) vehicle of Zymosan A® given intraperitoneally (SHAM); Group I (control), with Zymosan A® (0.6g/kg) intraperitoneally; Group II (Molsidomine), with Molsidomine® (4mg/kg) through the penis dorsal vein, 30min prior to administration of the Zy® (0.6g/kg); Group III (Celecoxib), with Celecoxib® (400mg/kg) orally through a stomach tube, 6h prior to administration of the Zy (0.6g/kg).
DeterminationsThe parameters survival, bacterial translocation, renal function, neutrophil accumulation, oxygen free radicals (OFR), detoxifying enzymes, and cytokines were measured at different times after Zymosan administration.
ResultsThe model established induced a mortality rate of 100% and generated BT and systemic inflammatory response syndrome (SIRS) in all samples. It also significantly increased all variables, with p<.001 for MPO and all pro-inflammatory cytokines, and p<.01 for all OFR. Treatment with Molsidomine reduced mortality to 0%, decreased BT, MPO, pro-inflammatory cytokines and OFR (p<.001) significantly and increased IL-10 and IL-6 production. Moreover, the Celecoxib® showed a lower capacity for SIRS regulation.
ConclusionsThe exogenous administration of NO prevented BT and controlled SIRS. Therefore these results suggest that Molsidomine could be used as a therapeutic strategy to protect against BT.
Evaluar los efectos beneficiosos del ON exógeno y de un inhibidor de la COX2 y sus niveles de acción en un modelo de síndrome de respuesta inflamatoria sistémica (SIRS)/traslocación bacteriana (TB) inducida por Zymosan A®.
Materiales y métodosNoventa ratas Wistar fueron sometidas a diferentes tratamientos, y después de 12 y 24 horas fueron anestesiadas para recoger sangre, nódulos linfáticos mesentéricos y tejido renal, para analizarlos bioquímica y microbiológicamente.
TratamientosUn donador de óxido nítrico, Molsidomina®, fue comparado con Celecoxib®, inhibidor de la COX2.
MétodosSe administró Zymosan A® a las ratas Wistar. Estas fueron divididas en CINCO grupos: grupo 1 (basal), sin manipulaciones; grupo 2 (sham), vehículo de Zymosan A® administrado intraperitonealmente; grupo I (control), con Zymosan A® (0,6 g/kg) intraperitoneal; grupo II (Molsidomina) con Molsidomina® (4 mg/kg) administrada a través de la vena dorsal del pene, 30 minutos antes de la administración de Zymosan® (0,6 g/kg); y grupo III (Celecoxib) con Celecoxib® (400 mg/kg) administrado oralmente por tubo esomacal, 6 horas antes de la administración de Zymosan A® (0,6 g/kg).
DeterminacionesSe midieron los parámetros supervivencia, traslocación bacteriana, función renal, acumulación de neutrófilos, radicales libres de oxígeno, enzimas detoxificantes y citoquinas, a diferentes tiempos después de la administración de Zymosan®.
ResultadosEl modelo establecido inducía una tasa de mortalidad del 100%, y se generaba traslocación bacteriana y síndrome de respuesta inflamatoria sistémica en todas las muestras. También se incrementaban significativamente todas las variables, con p < 0,001 para mieloperoxidasa y todas las citokinas proinflamatorias, y p < 0,01 para todos los radicales libres de oxígeno. El tratamiento con Molsidomina reducía la mortalidad al 0%, disminuía la traslocación bacteriana, mieloperoxidasas, citokinas proinflamatorias y radicales libres de oxígeno (p < 0,001), e incrementaba la producción de IL-10 e IL-6. Además, Celecoxib® mostró una menor capacidad para la regulación del síndrome de respuesta inflamatoria sistémica.
ConclusionesLa administración exógena de óxido nítrico evita la traslocación bacteriana y controla el síndrome de respuesta inflamatoria sistémica. Estos resultados sugieren que Molsidomina podría usarse como estrategia terapéutica frente a la traslocación bacteriana.
The underlying mechanisms of how and under what circumstances gastrointestinal bacteria translocate across the mucosal barrier have been extensively studied in several animal models.1–12 In fact, although bacterial translocation (BT) can be induced in a variety of animal models, it appears that at least one of three basic pathophysiologic factors must be present for it to occur:
- 1.
Disruption of the normal gut flora, resulting in a bacterial overgrowth with Gram-negative enteric bacteria.
- 2.
Physical disruption or impairment of the gut mucosal barrier.
- 3.
Impaired immune defenses of the host.
As clinical relevance, the same conditions documented to promote loss of gut barrier function and bacterial translocation in experimental models are commonly present in critically ill or injured patients. These patients frequently are immune suppressed. This situation may cause a systemic inflammatory response syndrome (SIRS),11,13–15 multiple organ failure (MODS),16–22 and even death.23–28 However, the exact nature of such relationships remains obscure.
In previous studies, it has been shown that SIRS could be crucial in the development of BT, and both SIRS and BT determine the progression to MODS if they are not well controlled.29–32
It is our aim to assess the SIRS and its consequences, due to BT and MODS with high mortality caused by Zymosan A® (Zy)® (protein–carbohydrate complex derived from Saccharomyces cerevisiae),30–34 as well as its control with Molsidomine® (nitric oxide donor)35–42 and Celecoxib® (inhibitor of the COX2)43,44 treatments, with the intention of increasing knowledge about the pathophysiology of these processes.
MethodsAnimals, experimental groups, and treatmentsAll animal procedures were in accordance with the guidelines for animal use published by Directive 2010/63/EU of the European Parliament, and with the Council of 22 September 2010 on the protection of animals used for scientific purposes and Spanish Government (B.O.E. No. 34, 08/02/2013, B.O.E. No. 268, 08/11/2007 and B.O.E. No. 140, 12/06/2013). The Bioethical Committee of the Salamanca University approved all experiments.
All adequate measures were taken to minimize animal pain or discomfort.
Healthy Wistar male rats (Charles River S.L, UK), weighing 250–275g, were fasted overnight but allowed free access to drinking water before surgery. Animals were anesthetized with ketamine hydrochloride (Parke-Davis, Morris Plains, NJ, USA) (75mg/kg)+diazepam (Valium, Roche, Spain) (50mg/kg)+atropine (Atropina, Braun, Spain) (20mg/kg) given intraperitoneally.
We develop an experimental model of MODS induced by inflammatory response and BT. Zy® (Sigma Chemical Co., St Louis, Missouri), according to Mainous et al.45 and other previous studies,30–32 promotes an unspecific and aseptic peritoneal systemic inflammation. We look for a dose of Zy® that causes a mortality between 80 and 90%.37,46–49 The dose of Zy® in this model was established in 600mg/kg diluted in 2mL of mineral oil and given intraperitoneally.
Subsequently, we evaluated the suitable doses of the treatments that should be used. Taking into consideration the previous experience of our group of investigation,30,50 we decide to use the clinical therapeutic doses of the above mentioned drugs: Molsidomine® 4mg/kg51 and Celecoxib® 400mg/kg.
Finally, we had to raise the most appropriate moment for the administration of the treatments. In light of the evolution of the inflammatory response in models of TB induced by Zy,27,52–55 and after assessing the survival of the animals previously studied, it was decided to apply the treatment at the following times:
Molsidomine® (4mg/kg), 30min prior to administration of Zy (600mg/kg) and Celecoxib® (400mg/kg), 6h before administration of Zy (600mg/kg).
Out of a total of ninety rats were randomly divided into 5 groups.
Under anesthesia and aseptic conditions shaved skin, sterile operative fields, and preparation with pavidone-iodine (Betadine®, Asta Médica), a medial laparotomy was carried out.
Group 1 (n=5): Basal. No manipulation. The rats were sacrificed to obtain the various tissues and blood samples.
Group 2 (n=5): Sham. Animals received 2mL of mineral oil (vehicle of Zy) intraperitoneally. After 12h, animals were anesthetized and samples of bacteriology mesenteric lymph nodes, kidney and blood were obtained.
Group I (n=10): Control. Animals received 2mL of mineral oil containing Zy 0.6g/kg, intraperitoneally. After 12 (moment of SIRS, according to previous studies) and 24h (situation of MODS, according to previous studies), animals were anesthetized and samples of bacteriology mesenteric lymph nodes, kidney and blood were obtained.
Group II (n=10): Molsidomine®. Animals received Molsidomine 4mg/kg body weight through the penis dorsal vein. After 30min, animals received 2mL of mineral oil containing Zy 0.6g/kg, intraperitoneally. After 12 and 24h animals were anesthetized and samples of bacteriology mesenteric lymph nodes, kidney and blood were obtained.
Group III (n=10): Celecoxib®. Animals received Celecoxib 400mg/kg body weight orally through a stomach tube. After 6h animals received 2mL of mineral oil containing Zy 0.6g/kg, intraperitoneally. After 12 and 24h animals were anesthetized and samples of bacteriology mesenteric lymph nodes, kidney and blood were obtained.
Animals having a delayed recovery from anesthesia or signs of hemorrhage were excluded from the study.
Variables, sample collection, and analysisUnder general anesthesia and aseptic conditions, the animals underwent a midline laparatomy. Samples of bacteriology mesenteric lymph nodes, kidney and blood were obtained.
Blood samples were collected by aortic puncture in heparinized sterile tubes containing EDTA, aprotinin (1.5mg/mL), and trypsin inhibitor (0.67units/mL). Plasma was separated by centrifugation at 10,000×g for 10min and stored at −80°C until measurements were performed.
Kidneys were removed and a portion of each rat's kidney was frozen in liquid nitrogen and conserved at −80°C. The remaining renal tissue was immediately used for superoxide anion (SOA) and myeloperoxidase activity (MPO) determination.
MicrobiologyTo evaluate the degree of BT, caecum and small bowel samples were taken from the Basal group. Once the flora had been identified in this group, mesenteric lymph nodes (MLN), blood (obtained by aortic puncture) and kidney samples from the other groups were examined to evaluate BT. All samples were labeled and immediately carried, at room temperature, to the laboratory. Aerobic and anaerobic (Gram-positive and Gram-negative) cultures were performed (Columbia blood agar; agar-chocolate supplied with isovitalex; Enterococcus agar supplemented with 10% biliary salts; salty manitol agar; Mac-Conkey campylosel cefoperazone, vancomycin and Amphotericin agar; HPA culture medium; vancomycin and nalidixic acid Wilkins-Chalgren blood agar; egg yolk agar and Sabouraud chloramphenicol gentamicin agar). Aerobic agar plates were incubated during 24–48h at 37°C, and anaerobic agar plates during 72ho at 37°C in anaerobic jars under the conditions of the GASPAK PLUS® anaerobic system (BBL Microbiology Systems, Becton-Dickinson and Co. Cockeyville, Maryland). Specific identification techniques (qualitative and quantitative) and graft sonication were performed.
Creatinine, creatinine clearance and ionic Na+, K+, Cl− concentrationsIn order to test renal function, animals were placed in metabolic cages and after 24h urine samples were collected. Blood was obtained from a cut in the tail tip of the heparinized capillaries. Ionic Na+, K+, Cl− concentrations were measured in an automatic analyzer Hitachi 747-200 (Boehringer Mannheim, Indianápolis, IN).
Creatinine urinary and plasmatic concentrations were determined using an automatized method based in the Jaffé reaction (Hitachi 717 automatic analyzer, reactives from Boehringer Mannheim, Manheim, Germany). Creatinine clearance was calculated using the conventional formulae.
Measurement of myeloperoxidase activityMyeloperoxidase (MPO) activity in kidney tissue was used as an index of renal neutrophil and macrophage infiltration. Renal samples obtained were processed, and MPO activity measured as previously described.51,56 Briefly, after tissue homogenization in 50mM Na-phosphate, 0.5% hexadecyltrimethylammonium bromide (Sigma, St. Louis, USA) and 0146% EDTA (Sigma) pH=6, samples were centrifuged during 30min at 15,000×g at 4°C. Supernatant was then incubated in a buffer containing 0.05% hydrogen peroxide, and MPO activity was measured at 460nm and 25°C. MPO activity (1 unit) was defined as the amount of protein able to degrade 1μmol of hydrogen peroxide/min at 25°C.
Renal tissue superoxide anionOxygen free radical (SOA) and detoxifying enzymes superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPX):
These parameters also reflect neutrophil activation through the NAD(P)H oxidase enzyme. Accordingly, together with the myeloperoxidase, the study of oxygen free radicals (OFRs) completed the evaluation of neutrophil activation/migration. Determination of the rate of synthesis of the free radical superoxide anion (SOA) was performed in liver and kidney using the modified Forman–Boveris technique.57 The activity of each defensive enzyme was also determined in liver and kidney using different techniques for SOD,51 CAT58 and GPX.59 Protein concentrations were measured spectrophotometrically by Bradford's method (reagents from Panreac and Sigma Chemical Co., St. Louis, Missouri).
Plasma cytokines (TNF-α, IL-1β, INF-γ, IL-6 and IL-10)Plasma levels of tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), interleukin-1β (IL-1β), interleukin-6 (IL-6), and interleukin-10 (IL-10) were measured using commercial enzyme-linked immunosorbent assay kits (ELISA), according to the manufacturer's instructions (R&D systems, Rat cytokines).
Data statistical analysisStatistical analysis was performed using the NCSS computer program.
Results are expressed as means±standard error of the mean (SEM). The exact Fisher test, Mann–Whitney U test, Kruskal–Wallis Z test and ANOVA (Student–Newman–Keuls) were used. Statistical significance was accepted for a value of p<0.05.
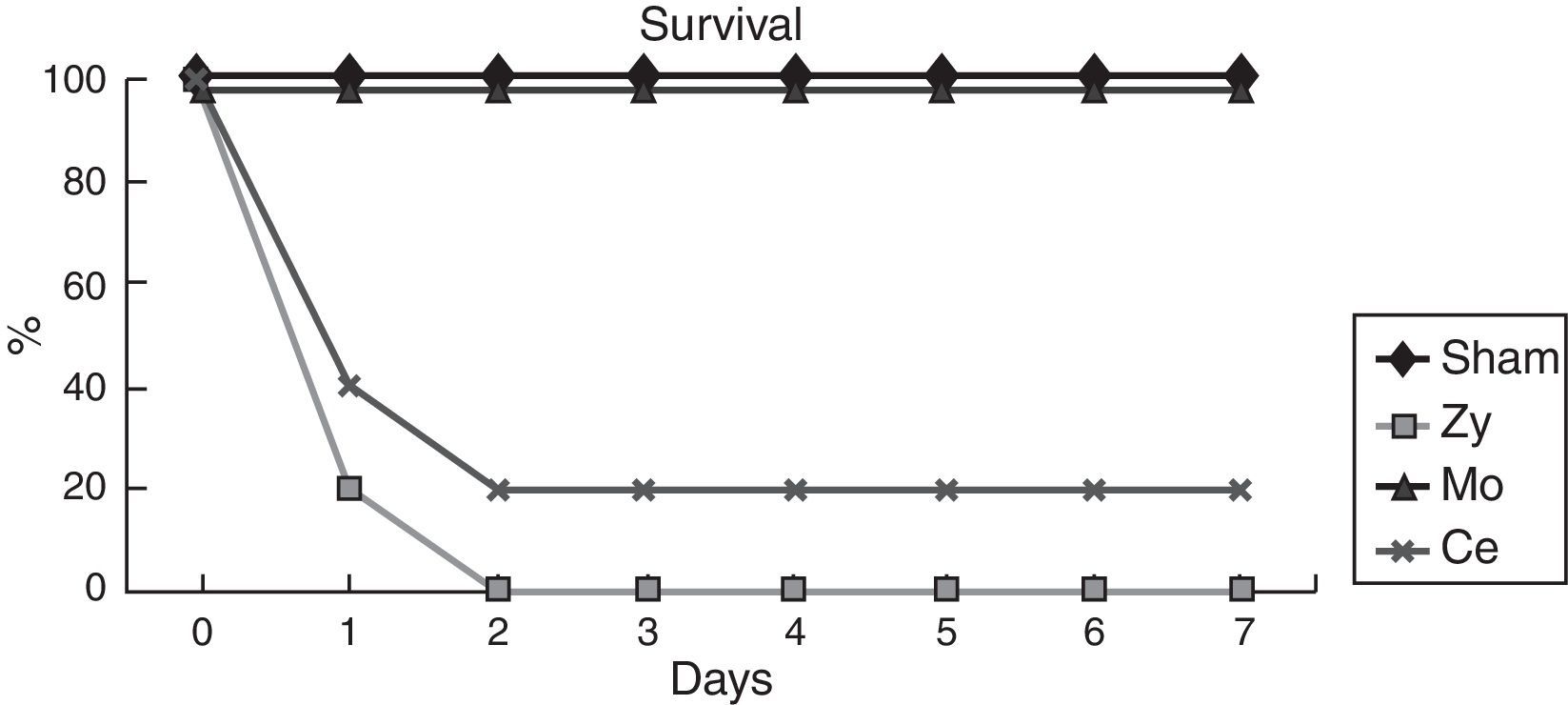
ResultsSurvivalIn order to examine the protective effect of Molsidomine®and Celecoxib®, a survival study was performed during 7 days. Survival was higher in the group receiving Molsidomine®(100%) than in the Celecoxib®(20%) group. In the Sham group no deaths occurred, being the survival rate at 7 days 100%, whereas 2 days after Zy administration Control group showed 100% mortality.
Microbiologic studyBacterial distribution in the caecum and small bowel in the Basal group (no injury) pointed to the constant presence of aerobic Gram-negative bacteria (Enterobacteriaceae). Escherichia coli was isolated systematically and some others only occasionally (Proteus mirabilis, Flavobacterium odoratum). Quantitatively, E. coli levels were between 0.01 and 5×105g−1 of feces. Gram-positive aerobic bacteria were also isolated systematically at levels of 0.2–6×106g−1 of feces, with a larger number of species (many species of Staphylococcus, Streptococcus, Corynebacterium, etc.). Anaerobic bacteria, mainly Bacteroides ovatus (0.005–1×106g−1 of feces), were also isolated.
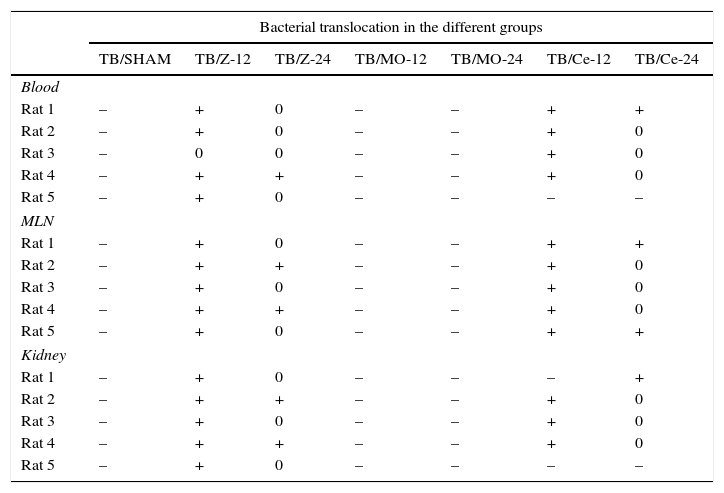
The Sham group did not exhibit any bacterial growth/contamination in the samples, showing that the mineral oil (Zymosan vehicle) had not induced BT and that our technique was correct. In the Zy group, a strong degree of affectation was observed, with BT affecting all samples collected from all animals and with statistical significance (p<0.001) with respect to the Sham animals. An increased presence of E. coli was noted in all tissues studied (Table 1). For the prophylactic treatment with Molsidomine® prevented BT, all samples were cultured with negative results, with statistical significance with respect to the Zy animals (p<0.001). For the prophylactic treatment with Celecoxib®, prevented BT, the significant differences with the Zy group animals (p<0.001) were confirmed (Table 1).
Bacterial translocation: number of samples with BT.
| Bacterial translocation in the different groups | |||||||
|---|---|---|---|---|---|---|---|
| TB/SHAM | TB/Z-12 | TB/Z-24 | TB/MO-12 | TB/MO-24 | TB/Ce-12 | TB/Ce-24 | |
| Blood | |||||||
| Rat 1 | – | + | 0 | – | – | + | + |
| Rat 2 | – | + | 0 | – | – | + | 0 |
| Rat 3 | – | 0 | 0 | – | – | + | 0 |
| Rat 4 | – | + | + | – | – | + | 0 |
| Rat 5 | – | + | 0 | – | – | – | – |
| MLN | |||||||
| Rat 1 | – | + | 0 | – | – | + | + |
| Rat 2 | – | + | + | – | – | + | 0 |
| Rat 3 | – | + | 0 | – | – | + | 0 |
| Rat 4 | – | + | + | – | – | + | 0 |
| Rat 5 | – | + | 0 | – | – | + | + |
| Kidney | |||||||
| Rat 1 | – | + | 0 | – | – | – | + |
| Rat 2 | – | + | + | – | – | + | 0 |
| Rat 3 | – | + | 0 | – | – | + | 0 |
| Rat 4 | – | + | + | – | – | + | 0 |
| Rat 5 | – | + | 0 | – | – | – | – |
MLN: mesenteric lymph nodes.
–: none.
+: Tb.
0: exitus.
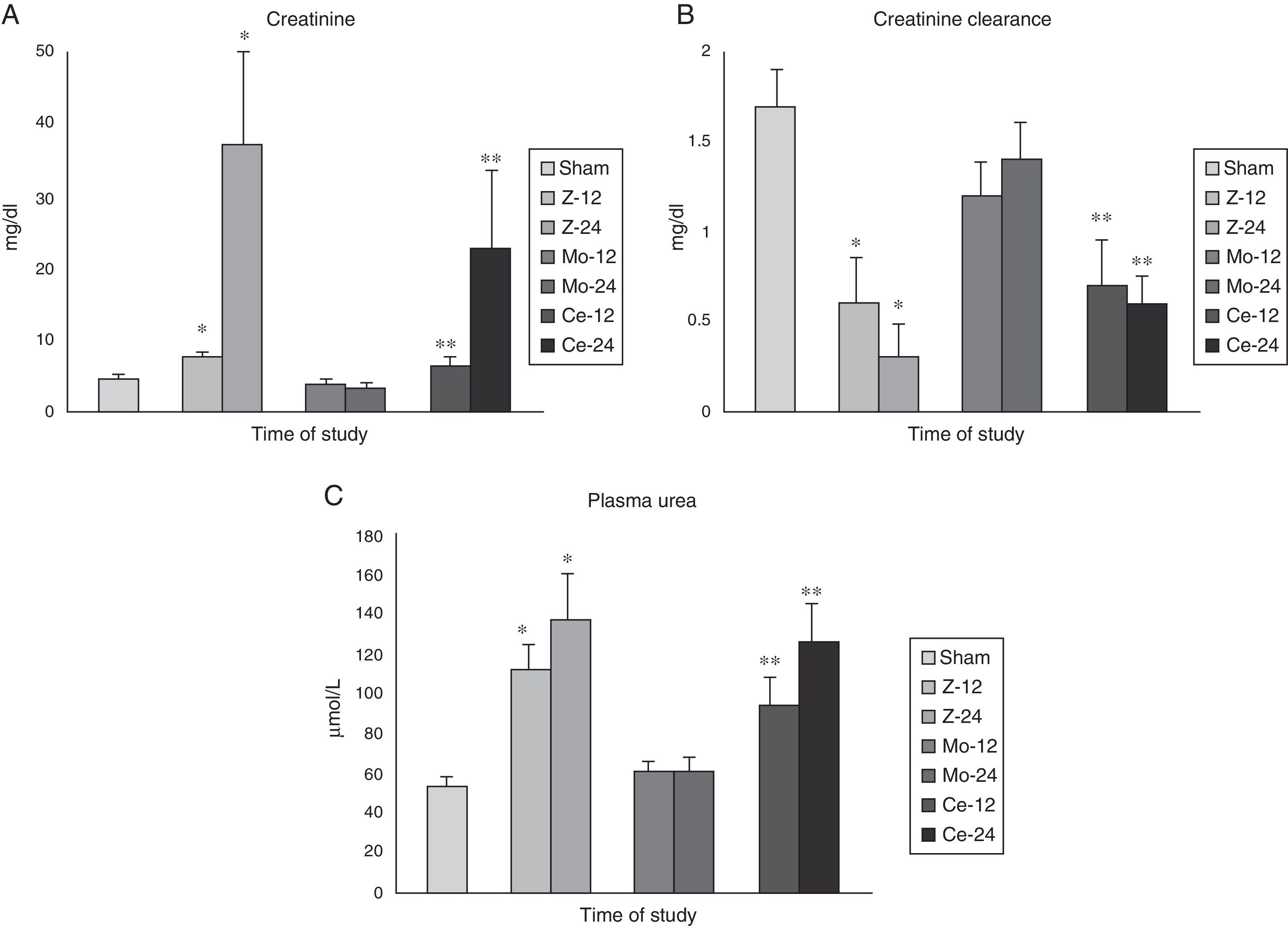
The study of the renal function demonstrated a serious alteration of it in the groups Zy (especially at 12h), being observed significant differences (p<0.001) among all the groups in the detected values of plasma creatinine, plasma urea and creatinine clearance.
The Celecoxib® group presented a maintenance of the renal function according to the studied parameters. In case of the Molsidomine® group an improvement of the function (p<0.05) was observed with regard to the Zy group (Fig. 1).
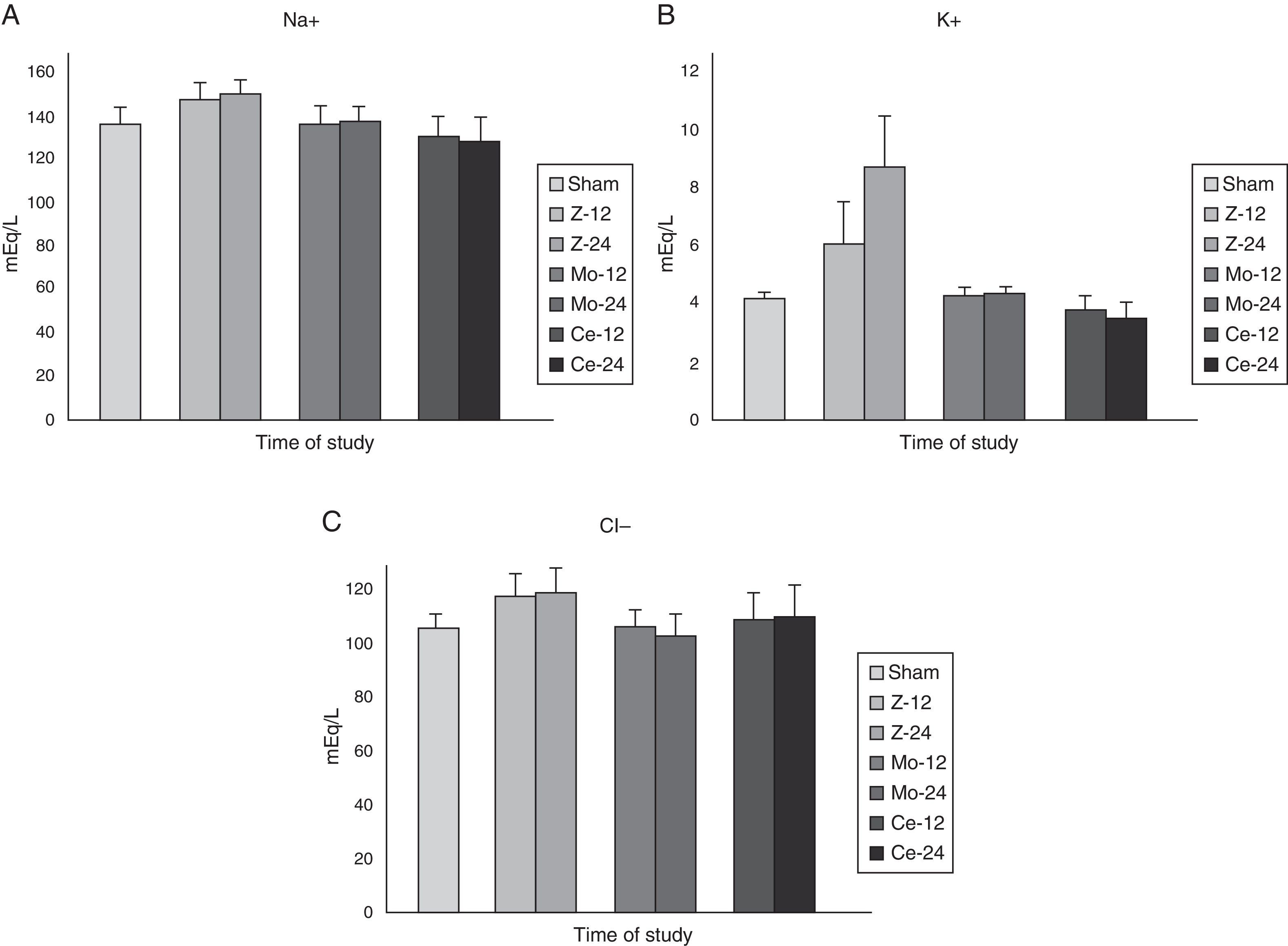
In spite of the serious alterations detected in the renal function, the values observed of the ionic (Na+, K+, Cl−) study did not present significant differences among the different studied groups (Fig. 2).
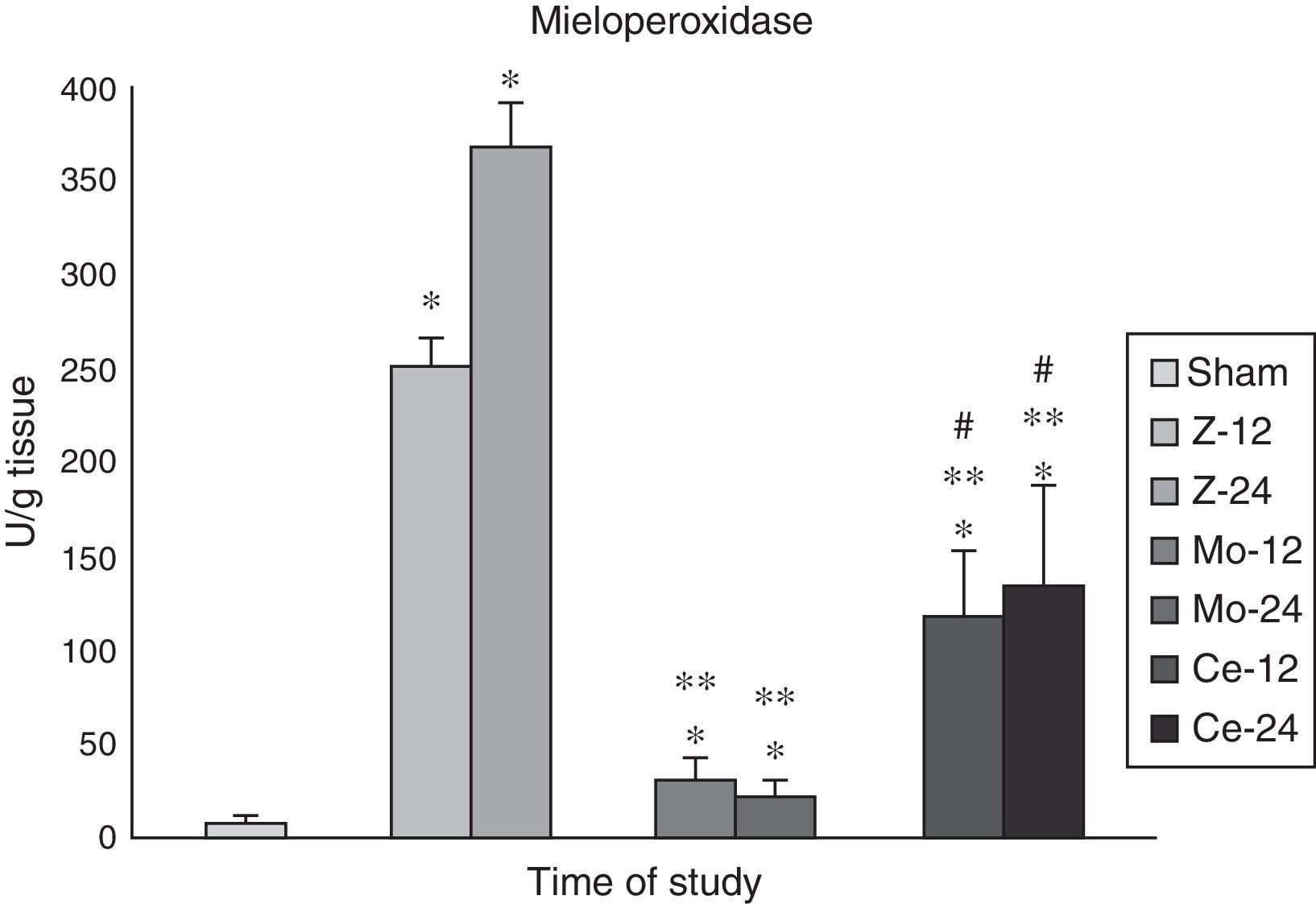
Myeloperoxidase (MPO)As neutrophil and macrophage infiltration is a key step in the tissue inflammation induced by Zy, mieloperoxidase (MPO) activity was measured in renal tissue.
The aggressions in the Zy group promoted a remarkable increase in MPO renal levels (p<0.001) in comparison with the Sham animals, reflecting neutrophil infiltration.
In Molsidomine® group, these levels decreased significantly (p<0.05).
In Celecoxib® group, the levels of this enzyme detected higher than in the Molsidomine® group (p<0.01) (Fig. 3).
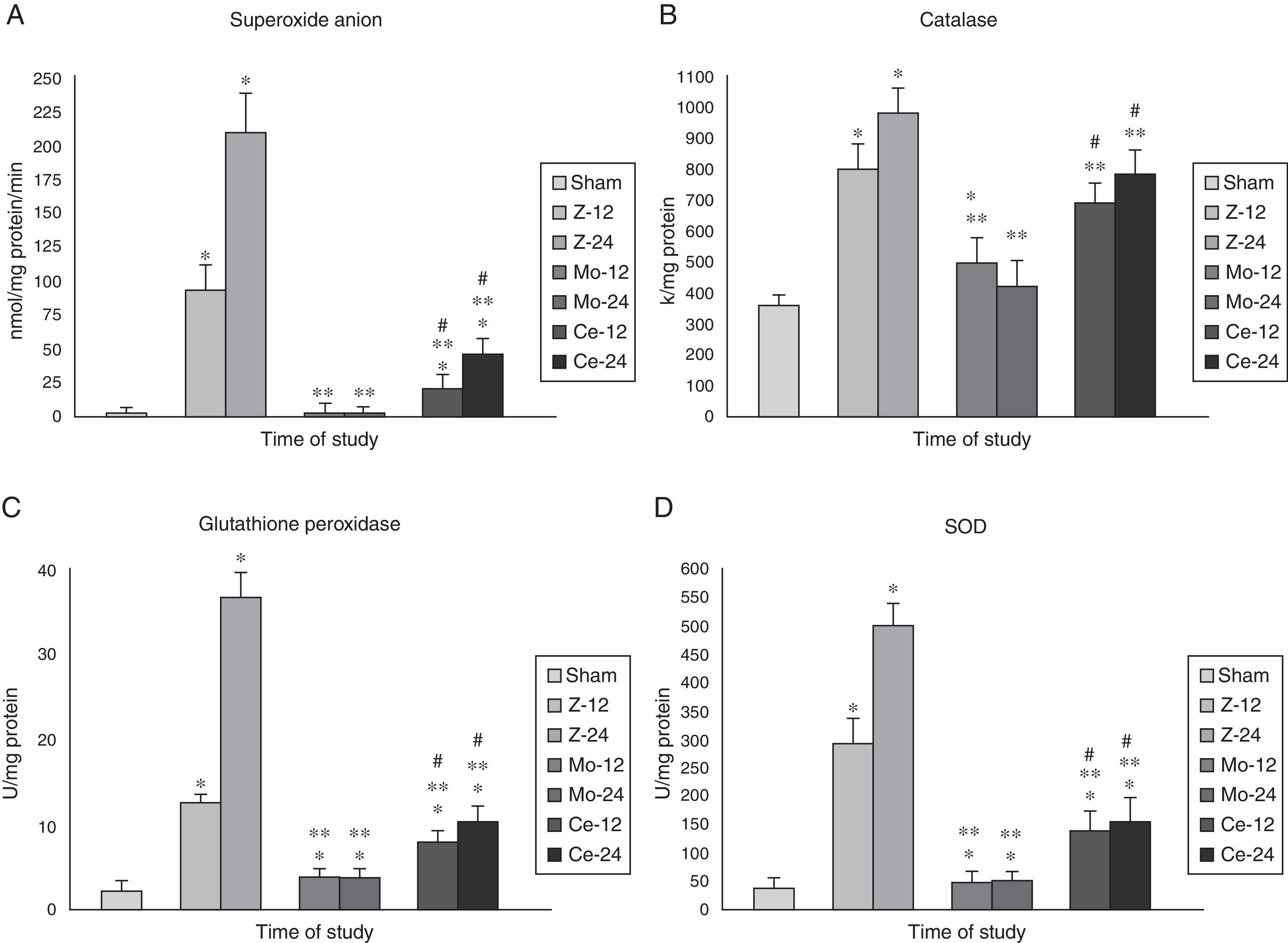
Superoxide anion (SOA) and defensive enzymes (SOD, CAT, GPX)Renal levels of superoxide anion (SOA) after Zy administration were markedly higher in the Zy group than in the Sham, Molsidomine and Celecoxib groups (Fig. 4A).
Mieloperoidase. Results for MPO in kidney tissue. Note that maximum significance is reached at 24h post-Zymosan. The comparisons of other groups are also significative at each time, respectively. Values are expressed as mean±SEM. * indicates significant at p<.001 vs Sham group, ** indicates significant at p<.001 vs Zy group, and # indicates significant at p<.001 vs Molsidomine group.
Renal levels of Defensive Enzymes behaved in the same way, with a significant increase (p<0.01) in the Zy group as compared to the Sham animals being observed.
In the Molsidomine® group, defensive enzymes activity significantly decreased in comparison with the Zy animals (p<0.01). Treatment with Celecoxib® also showed decreases in these variables’ levels, although they were less prominent than those found for the Molsidomine® group (p<0.05) (Fig. 4B–D).
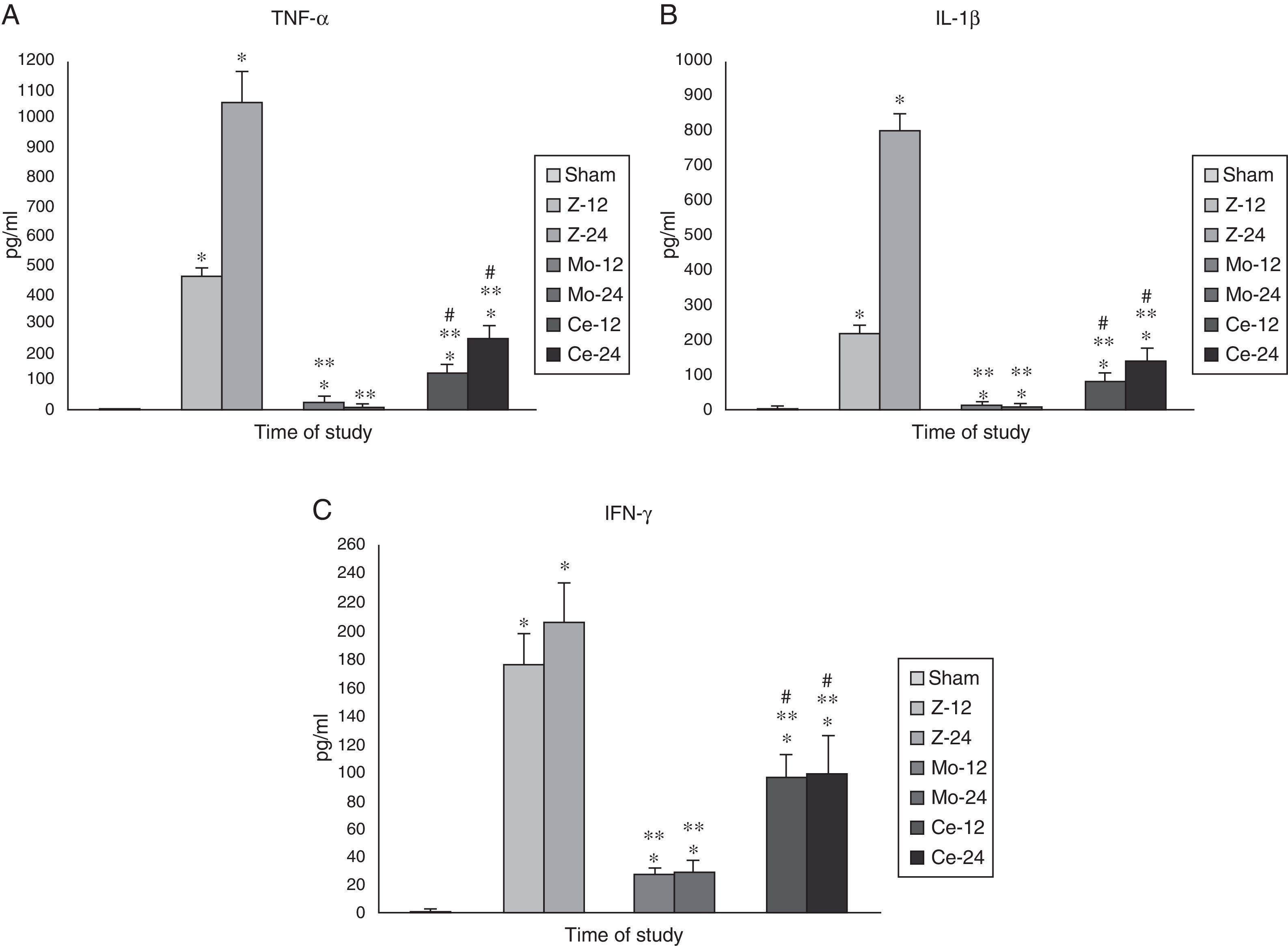
Inflammation markers (TNF-α, IFN-γ, IL-1β, IL-6 and IL-10)Plasma levels of tumor necrosis factor (TNF-α), interferon-γ (IFN-γ), interleukin-1β (IL-1β), were significantly higher in the Zy group than in the Sham group. Treatment with Molsidomine® blocked the elevation of these three cytokines
Celecoxib® group also showed decreases, although they were much less prominent than those found for the Molsidomine® group (Fig. 5A–C).
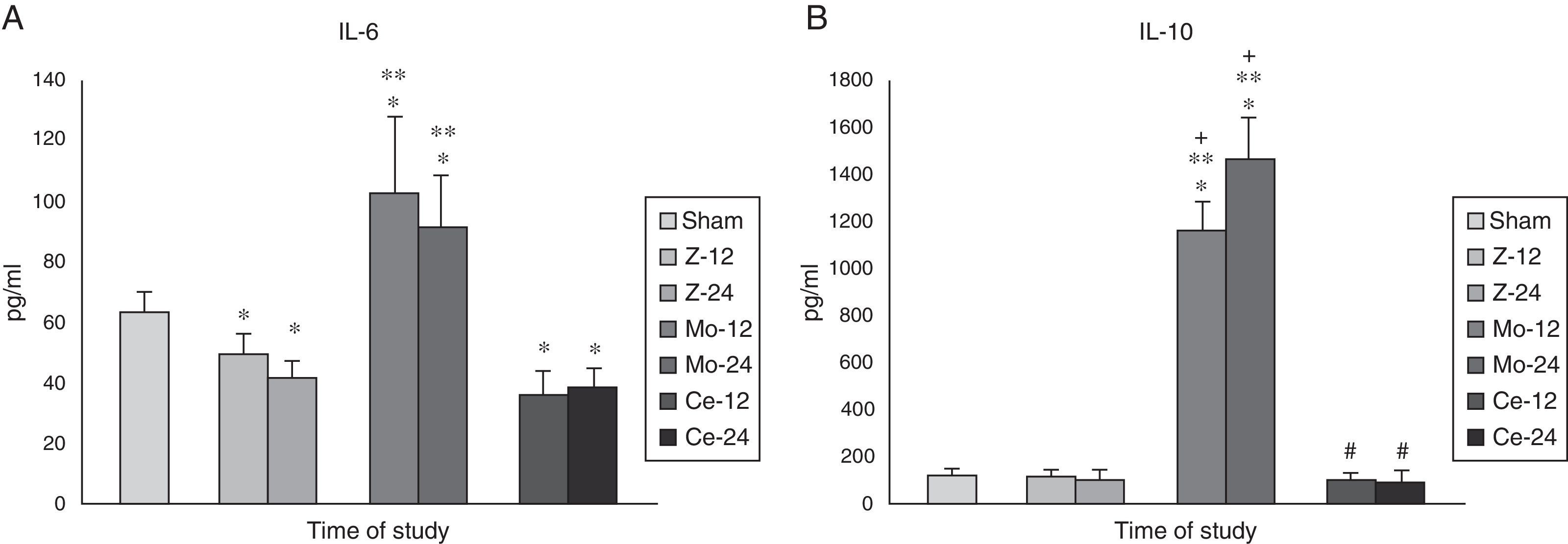
Plasma levels of IL-6 and IL-10 were significantly higher in the group treated with Molsidomine® than those observed in the Sham, Zy and Celecoxib groups. No significant differences in IL-6 and IL-10 levels were observed between animals with Celecoxib and Zy (Fig. 6A and B).
DiscussionIt is known that BT is able to initiate SIRS, or more probably to enhance it (in a second phase).26,60 Being that translocated bacteria participate in SIRS, they are also likely to contribute to MODS and the consequent death of critically ill patients.27,61 However, the exact role of BT in the pathogenesis of MODS is not completely clarified.62 Many authors have advocated against such a relationship.53 Nevertheless, a clear relationship between BT, SIRS and MODS was observed in our model.
The aim of the present work was to attempt to modulate the systemic inflammatory response and to prevent BT in a situation of aggression. It is clear that SIRS, as an exaggerated and harmful inflammatory response of the organism to an aggression, promotes BT.63 SIRS can be defined as a physiological phenomenon characterized by a sepsis-like condition, present in many clinical and surgical complications, for which no infectious source can be demonstrated.64,65
Our data demonstrate that the administration of Molsidomine® to rats clearly improved animal survival compared with animals that received only Zy. In addition, renal function assessed by plasma (creatinina, urea) and creatinine clearance was better in the Molsidomine® group38–41 than in the Zy group.30 During an acute inflammatory response, a complex cascade of mediators is triggered to recruit neutrophils to the place of tissue injury.66,67 In many tissues a number of stimuli (such as shear stress, LPS, ischemia-reperfusion, immune complexes) may result in neutrophil recruitment/adhesion to endothelium surface, where they start to produce cytokines and oxygen free radicals.42,67 All variables determined increased in all samples, and death occurred in 100% of the animals in the Zy group. The model was based on sequential insults that, as in human clinical situations, lead precisely (due to the exacerbation of SIRS) to multiple organ dysfunction syndrome (MODS). The effect of exogenous NO administration to that group resulted in a positive SIRS modulation in nearly all the variables studied, values that were not significantly different from those of the Sham group. Celecoxib® group also showed decreases, although they were much less prominent than those found for the Molsidomine® group. The mechanism involved in this protection is probably multifactorial, including a reduction of oxidative stress and the anti-inflammatory properties of Molsidomine®.39,68 The effect of downregulating pro-inflammatory cytokines such as TNF-α, IL-1-β and IFN-γ can also explain, at least in part, the decrease in OFRs, since these latter were stimulated by cytokine action. Furthermore, the plasma levels of IL-6 and IL-10, are higher in Molsidomine® and Celecoxib® groups than in Zy group. These higher levels of IL-6 and IL-10 could also participate in the protecting effect of Molsidomine® and Celecoxib®. These effects of Molsidomine seem to be mediated by a reduction in the oxygen-radical production, an attenuation of neutrophil and macrophage infiltration, a lower inflammation demonstrated by a decrease of plasma levels of pro-inflammatory cytokines secretion such as TNF-α, IL-1β and IFN-γ, and a higher IL-10 and IL-6 production (Fig. 7).
In conclusion, our results demonstrate that the experimental model and design developed, was valid for the study aims. Zymosan induces a SIRS that induce BT and MODS with high mortality rate. The use of an exogenous NO donor (Molsidomine) reduces/eliminates translocated bacteria and modulates SIRS.42,67
Celecoxib® also showed control of BT and modulation of SIRS, although was much less prominent than those found for the Molsidomine group.
Conflict of interestThe authors declare no conflict of interest.