NICE guidance in 2009 recommended oseltamivir treatment to all hospitalized children with suspected flu in the epidemic weeks, based on the possibility of influenza infection in children with compatible symptoms, is about 58%.1 Recognizing that this may be overestimating the rate of influenza, they recommend further research into the probability that an influenza-like illness is true influenza. Since then, the recommendations have remained virtually unchanged.2 The American Academy of Pediatrics (AAP) has also, this year, recommended treatment for all hospitalized children.3 Rapid detection influenza tests have moderate sensitivity (50–70%). Therefore, antiviral treatment has been recommended even in cases with negative laboratory results. Oseltamivir has demonstrated to reduce the duration of symptoms, especially if it is administrated in the first 48h of the illness with mild or inexistent side effects. Its role to prevent complications is not enough proven. Summarizing, emergence of antiviral resistance is an important clinical and public health concern.4
In the Pediatric Department at the Severo-Ochoa Hospital in Spain, we conducted a prospective study of viral etiology of respiratory infection during seven consecutive seasons. Between December and February each season (12 weeks that included the epidemic peak), all children under 14 years hospitalized with criteria of suspected flu (febrile syndrome, upper or lower respiratory tract infection, bronchiolitis, wheezing episodes or pneumonia), were included in the study. In the 2009–10 season, patients were recruited from September to December, coinciding with the H1N1 pandemic. A total of 1612 cases were analyzed. Polymerase chain reaction for 17 respiratory viruses in nasopharyngeal aspirate was performed in the Respiratory Virus and Influenza Unit at the National Microbiology Center (ISCIII, Madrid, Spain).
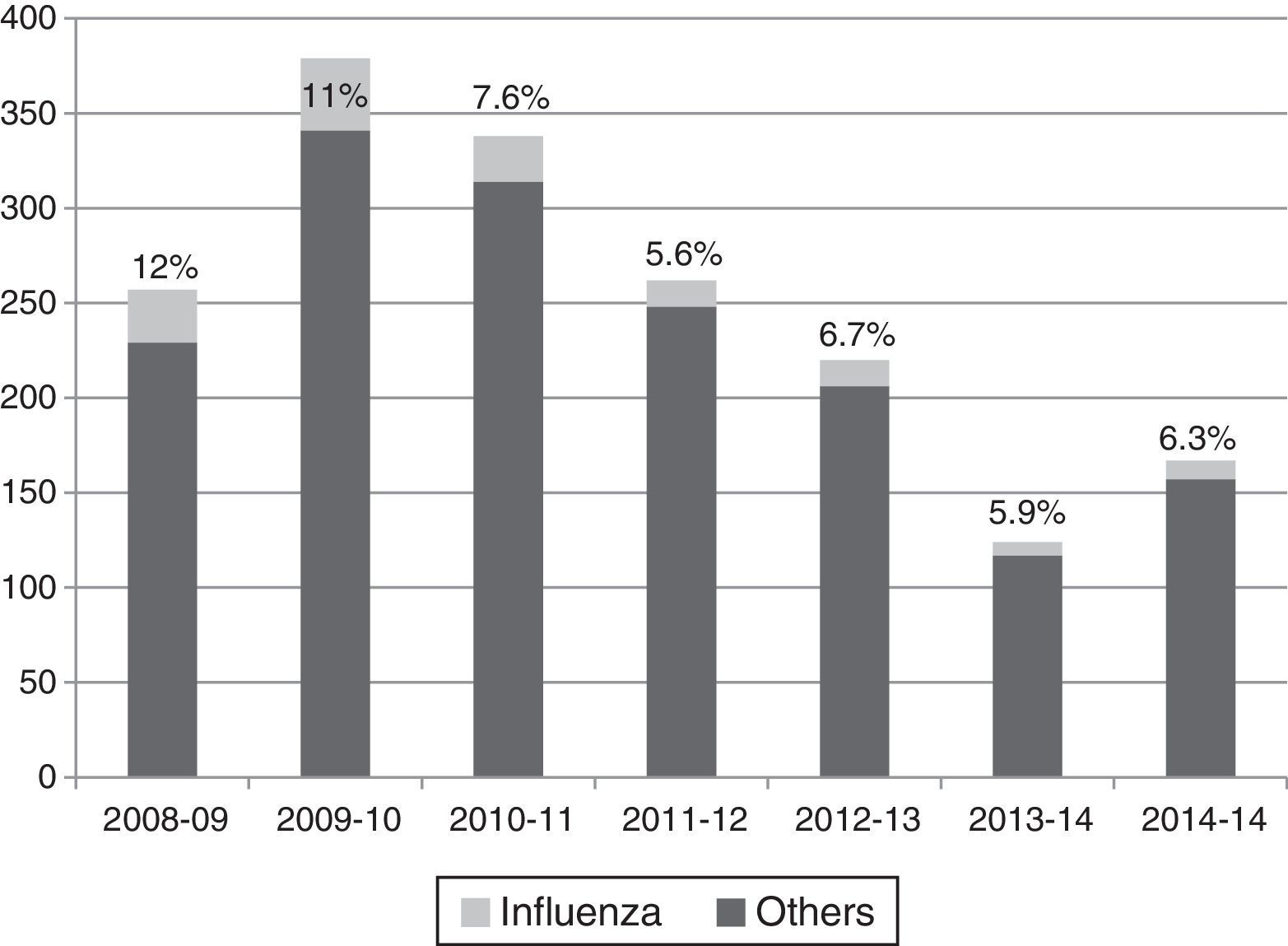
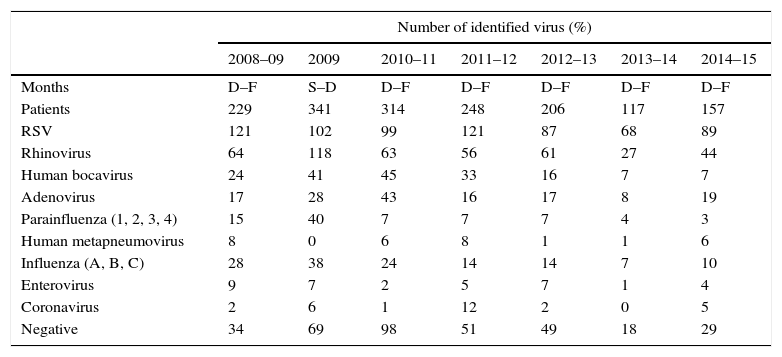
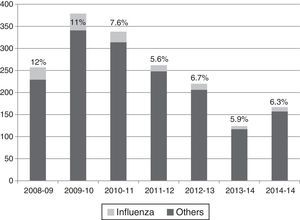
Influenza viruses were detected in the 5.6–12% of cases, depending on the season, with the highest incidence corresponding to the H1N1 pandemic season (Fig. 1). The proportion of different viruses detected is shown in Table 1, being respiratory syncytial virus the most frequent. Following the current recommendations, 1477 children without influenza virus confirmed by laboratory, would had been treated with oseltamivir.
Viruses detected by polymerase chain reaction in hospitalized children in the flu epidemic weeks.
| Number of identified virus (%) | |||||||
|---|---|---|---|---|---|---|---|
| 2008–09 | 2009 | 2010–11 | 2011–12 | 2012–13 | 2013–14 | 2014–15 | |
| Months | D–F | S–D | D–F | D–F | D–F | D–F | D–F |
| Patients | 229 | 341 | 314 | 248 | 206 | 117 | 157 |
| RSV | 121 | 102 | 99 | 121 | 87 | 68 | 89 |
| Rhinovirus | 64 | 118 | 63 | 56 | 61 | 27 | 44 |
| Human bocavirus | 24 | 41 | 45 | 33 | 16 | 7 | 7 |
| Adenovirus | 17 | 28 | 43 | 16 | 17 | 8 | 19 |
| Parainfluenza (1, 2, 3, 4) | 15 | 40 | 7 | 7 | 7 | 4 | 3 |
| Human metapneumovirus | 8 | 0 | 6 | 8 | 1 | 1 | 6 |
| Influenza (A, B, C) | 28 | 38 | 24 | 14 | 14 | 7 | 10 |
| Enterovirus | 9 | 7 | 2 | 5 | 7 | 1 | 4 |
| Coronavirus | 2 | 6 | 1 | 12 | 2 | 0 | 5 |
| Negative | 34 | 69 | 98 | 51 | 49 | 18 | 29 |
RSV: respiratory syncytial virus, D: December, F: February, S: September.
The number of total virus is superior to patients because the presence of co-infections.
As other authors, we think that the greater value of using oseltamivir is to ensure it remains an effective defense against future seasonal and pandemic influenza viruses. Careful monitoring of levels of viral resistance in the circulating viruses combined with the further development of new anti-influenza drugs might be the best way for control.5 Based on our personal experience, we recommend making an effort to improve diagnosis in children with suspected influenza, performing molecular techniques or a rapid diagnostic test with high sensitivity,6 especially in children with risk factors and children requiring hospitalization. This may help to guide treatment with oseltamivir to patients who really need it.
This study has been partially supported by FIS (Fondo de Investigaciones Sanitarias – Spanish Health Research Fund) Grants N°: PI06/0532, PI09/00246, and PI12/01291.