Diagnosis of HSV-1 keratitis (HK) is frequently based on clinical findings. Invasive specimens (corneal scrapings, biopsies) are required for microbiological diagnosis.
MethodsCorneal scrapings and conjunctival swabs were collected on patients with/without clinical suspicion of HK from 2007 to 2012.
ResultsThe sensitivity, specificity, positive and negative predictive values for conjunctival swabs by PCR was 77.8, 92.1, 84.4 and 88.3, respectively.
DiscussionConjunctival swabs by PCR may help in the diagnosis of HK, despite the limited sensitivity.
El diagnóstico de queratitis herpética (QH) está basado normalmente en hallazgos clínicos. Para el diagnóstico microbiológico se requieren muestras invasivas (raspado corneal, biopsias).
MétodosRaspados corneales y exudados conjuntivales fueron obtenidos de pacientes con/sin sospecha clínica de QH del año 2007 al 2012.
ResultadosLa sensibilidad, la especificidad y los valores predictivos positivos y negativos para la PCR en exudados conjuntivales fueron 77,8, 92,1, 84,4 y 88,3, respectivamente.
DiscusiónLa PCR en exudados conjuntivales puede ayudar en el diagnóstico, a pesar de su limitada sensibilidad.
Keratitis is a relatively common eye disease, where a wide range of microorganisms, including bacteria, fungi, protozoa, and viruses, have been described as infectious agents. Among these are Herpes simplex virus type 1 (HSV-1), which is a prevalent viral pathogen infecting the majority of the population,1 and is the leading infectious cause of corneal blindness worldwide.2 Despite the diagnosis of HSV-1 keratitis is frequently based on clinical findings, there are many of atypical cases where laboratory testing may be indicated for accurate diagnosis.3 Corneal scrapings (CS) or biopsy specimens are required as clinical samples; however, this kind of specimens demand invasive techniques and sometimes is impossible to achieve. Virus isolation, immunofluorescent staining or the polymerase chain reaction (PCR) has been used to identify HSV-1 on the ocular surface.4 Virus isolation is considered the gold standard procedure for the diagnosis of viral infections,5 but it takes several days to obtain results because of the small amount of material that can be obtained from these types of specimens.3 Prompt and rapid diagnostic tools are needed for an early diagnosis and adequate therapy, for the purpose of improving the outcome of patients.
The first aim of this study was to evaluate the usefulness of PCR as a rapid diagnostic method compared with the viral culture, and to assess if conjunctival swabs (CSW) samples were equivalent to corneal scrapings to diagnose herpetic keratitis (HK).
MethodsA prospective study was undertaken on patients with clinical suspicion of HK (epithelial keratitis) and with non-herpetic (nHK) corneal ulcer from June 2007 to June 2012, treated at the emergency department of Hospital Universitario 12 de Octubre (Madrid, Spain). CSW were obtained by polyester swabs and CS by a platinum Kimura spatula using as viral transport medium UTM (Universal transport medium, Copan Diagnostic, Inc.). All samples, after decontamination, were inoculated on A549 and MRC-5 cell lines (Vircell, S.L., Genoma, Spain), incubated at 37°C and evaluated for cytopathic effect development for seven days. The viruses were classified by immunofluorescence assay (IFA) with monoclonal antibodies for HSV-1 and HSV-2 antigen (Micro Track HSV1/HSV2 Culture Identification/Typing Test (Trinity Biotech, Wicklow, Ireland)). A total of 500μl of each sample was extracted using Nuclisense EasyMag® instrument (bioMérieux, Marcy l’Etoile, France) according to the manufacturer's instructions. The set of primers and Taqman probes used to detect HSV-1 and HSV-2 by a real-time singleplex PCR were previously described.6 The amplification was conducted in a LightCycler® 2.0 real-time PCR system (Roche Diagnostic, Spain). Univariate analysis was performed by using the t test for continuous variables and the χ2 or Fisher exact tests for categorical variables.
ResultsOver the study period, 188 patients were sampled. Sixty-seven (35.6%) patients were clinically diagnosed as HK with an average (±SD) of 56.9 (±19.7) years of which 52.2% (35/67) were men. Corneal scrapings from 49 (73.1%) patients were positive, 14 (20.9%) negative and 4 (6%) samples presented Taq polymerase inhibitors; and by viral culture, 31 (46.2%) patients were positive. Thirty-two (47.8%) CSW samples were positive, 35 (52.2%) negative and none was inhibited by PCR, while by cell culture, only 19 (28.3%) patients diagnosed as HK were confirmed.
One hundred and twenty-one patients were diagnosed as nHK, with an average of 57 (±21.6) years with 54.4% (66/121) as women. Nine (7.4%) CS were positive, 106 (87.6%) negative and 6 (4.9%) inhibited by PCR; and by cell culture, 3 (2.4%) positive and 118 (97.6%) negative. Three (2.4%) CSW were positive, 117 (96.7%) negative and 1 (0.9%) inhibited by PCR, while by cell culture, 2 (1.6%) were positive and 119 (98.4%) negative.
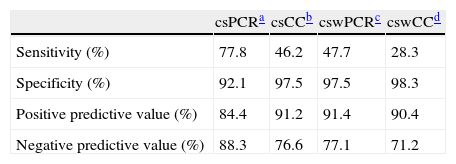
Sensitivity, specificity, positive and negative predictive values when the clinical diagnosis of HK was compared with the different methods such as corneal scraping PCR (csPCR), cell culture corneal scraping (csCC), conjunctival swabs PCR (cswPCR) and cell culture conjunctival swabs (cswCC) are shown in Table 1. The distribution of patients diagnosed as HK according to different techniques and samples is shown in Fig. 1. Four samples were inhibited when csPCR was performed (all of them were negative by cell culture), while the CSW obtained at the same time, 2 were positive, 2 negative and none of the cswPCR were inhibited.
Sensitivity, specificity, positive and negative predictive values of different diagnostic methods and samples.
When csPCR was compared with cswPCR, 29 samples were positive by both methods while 13 were negative, 20 were only positive by csPCR and 1 was only positive by cswPCR. Regarding viral culture, 19 were positive by both methods, 36 were negative, and 12 positive by csCC. Then, cswPCR was able to diagnose 59.2% (29/49) from patients confirmed from clinical diagnosis of HK by csPCR and 43.3% (29/67) with clinical diagnosis of HK. The agreement of CS and CSW was 66.7% [k=0.35 (0.16–0.53), p=0.0006] for real time PCR assay and 81.4% [k=0.63 (0.45–0.80), p<0.005] for cell culture, while that of PCR and viral culture for both samples was 71.43% [k=0.43 (0.25–0.61), p<0.005] and 80.6% [k=0.60 (0.42–0.78), p<0.005], respectively.
In patients with clinical diagnosis of HK who were negative for all trials, no other microorganism was detected. However, other microorganisms were isolated in 19 patients without clinical diagnosis of HK: Staphylococcus aureus (n=8), Streptococcus pneumoniae (n=4), Pseudomonas aeruginosa (n=2), Streptococcus viridians group (n=2), Haemophilus influenzae (n=2) and Candida albicans (n=1).
DiscussionThe diagnosis of some forms of presentation of the ocular HSV can be done through the clinical manifestations; however, there are many rare cases in which diagnosis could be confused with other etiologies leading to erroneous treatments and delay healing. The gold standard of HSV detection is viral culture although it is of very low sensitivity and time-consuming.7 This study was performed to evaluate PCR vs viral culture and to compare CSW vs CS as less invasive specimen in the diagnosis of HK. Compared to clinical diagnose, PCR sensitivity was higher than cell culture (77.8% vs 46.2%) when it was compared to clinical diagnose and it was able to diagnose 73.1% of suspected HK. Then, these results confirmed that PCR must be considered as gold standard instead of cell culture, as well as other studies, which reported high detection rates with sensitivities ranging from 70 to 100% depending on the study design.3,5,7–9
Numerous results have been published which compared different samples to diagnose HK (corneal scrapings, tear films, saliva, cornea tissues, aqueous humor and trigeminal ganglia).3,7–14 csPCR detected 25.3% more positive cases than cswPCR, the latter being comparable with csCC, which was able to detect 26.9% fewer cases. For which, CSW allowed to detect HSV-1 DNA in 60% of positive CS samples, rate similar to another study in which 50% of positive cases by csPCR, were positive using tear films as specimen, while csCC only detected 6 cases more than when it was used tear films. However, a high rate of 75% was detected by using tear films as specimen in a Spanish study.15 Despite corneal scraping being the clinical specimen of choice for laboratory diagnosis, 20.9% of the CS obtained from patients with clinical suspicion of HK were found negative by PCR, pointing out the need of an adequate sample with enough material.
Regarding 121 non-herpetic keratitis patients, only a total of 9 (7.4%) patients had asymptomatic shedding by csPCR and 3 (2.4%) by cswPCR, obtaining values much lower in contrast with other studies which highlighted high frequency (90%) of HSV-1 shedding in human subjects without herpetic ocular diseases as well as the finding that 90% of human trigeminal ganglia contains HSV-1 DNA.11,14,16 These differences could be possibly explained because of the number of samples taken over (1 sample per subject), since others authors have collected 60 samples per subject11 contributing to an unacceptable rate of false positive when testing was performed on tear samples obtained from healthy subjects. As a result of our findings, we considered that CSW are useful samples in the diagnosis of typical HK, although for atypical ocular presentations of the disease it must be taken into account the likelihood that in a few cases a positive result could be due to asymptomatic shedding.
In summary, these results confirmed that PCR must be considered the gold standard method in the diagnosis of typical HK. Conjunctival swabs allowed the detection of HSV-1 in 60% of positive corneal scraping samples, for which, it may help and serve as a supplemental method for diagnosis of typical HK despite limited sensitivity when the collection of CS would not be feasible.
Conflict of interestThe authors declare no conflict of interest.